Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans
- PMID: 20099249
- PMCID: PMC11742174
- DOI: 10.1002/cyto.a.20859
Evaluation of a 12-color flow cytometry panel to study lymphocyte, monocyte, and dendritic cell subsets in humans
Abstract
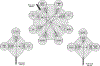
Monitoring changes in human immune cell populations such as lymphocytes, monocytes, and dendritic cells (DCs) during infectious diseases like human immunodeficiency virus (HIV) is crucial. However, difficulties to identify rare or heterogeneous cell populations can be limiting. For example, to accurately measure DC subsets, eight flow cytometry parameters are ideal. The aim of this work was to analyze the phenotype of human lymphocyte, monocyte, and DC subsets using a single 12-color flow cytometry panel. After erythrocyte lysis, blood from healthy human volunteers was washed and labeled with a cocktail of 12 antibodies. Samples were analyzed on a Becton-Dickinson FACSAria equipped with three lasers. Data were compared with lineage-specific panels using 5-8 Ab combinations per lineage. Acquired data were analyzed using FlowJo software. Our 12-color panel allows for the identification of the following major subsets of circulating cells in a single tube: CD4+ and CD8+ T lymphocytes, B lymphocytes, NK cells, NKT cells, monocyte subsets (CD14 and/or CD16), and five nonoverlapping HLA-DR+Lin- subsets: CD34+ hematopoietic stem cells, CD123+ plasmacytoid DC, and three subsets of CD11c+ myeloid DC expressing either CD16, CD1c (BDCA-1), or CD141 (BDCA-3). We have developed a single flow cytometry panel that allows for simultaneous detection of the lymphocyte and monocyte cell populations and all known DC subsets. Studying these major players of the immune system in one single panel may give us a broader view of the immune response during HIV infection and the ability to better define the role of individual cell types in Acquired Immune Deficiency Syndrome (AIDS) pathogenesis. (c) 2010 International Society for Advancement of Cytometry.
Figures

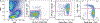



References
-
- Stevenson M HIV-1 pathogenesis. Nat Med 2003;9:853–860. - PubMed
-
- Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005;434:1093–1097. - PubMed
-
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 1999;283:857–860. - PubMed
-
- Zhang D, Shankar P, Xu Z, Harnisch B, Chen G, Lange C, Lee SJ, Valdez H, Lederman MM, Lieberman J. Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood 2003;101:226–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

