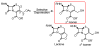
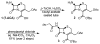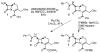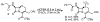Enzymatic deprotection of the cephalosporin 3'-acetoxy group using Candida antarctica lipase B
- PMID: 20099862
- PMCID: PMC2824665
- DOI: 10.1021/jo902406b
Enzymatic deprotection of the cephalosporin 3'-acetoxy group using Candida antarctica lipase B
Abstract
Cephalosporins remain one of the most important classes of antibiotics. A useful site for derivatization involves generation of and chemistry at the 3'-hydroxymethyl position. While 3'-acetoxymethyl-substituted cephalosporins are readily available, deacetylation to access the free 3'-hydroxymethyl group is problematic when the carboxylic acid is protected as an ester. Herein we report that this important transformation has been efficiently accomplished using Candida antarctica lipase B. Although this transformation is difficult to carry out using chemical methods, the enzymatic deacetylation has been successful on gram scale, when the cephalosporin is protected as either the benzhydryl or tert-butyl esters and on the corresponding sulfoxide and sulfone of the tert-butyl ester.
Figures
Similar articles
-
Synthesis of Ribose - Oleic Acid Esters in the Presence- and Absence of Candida antarctica Lipase B.J Oleo Sci. 2020 Aug 6;69(8):907-912. doi: 10.5650/jos.ess20086. Epub 2020 Jul 9. J Oleo Sci. 2020. PMID: 32641616
-
Candida antarctica lipase-B-catalyzed kinetic resolution of 1,3-dialkyl-3-hydroxymethyl oxindoles.Chirality. 2020 Dec;32(12):1377-1394. doi: 10.1002/chir.23284. Epub 2020 Nov 3. Chirality. 2020. PMID: 33141985
-
Cephalosporin 3'-phloroglucide esters and 7-(phloroglucidamido) cephalosporins as novel antibacterial agents.J Med Chem. 1997 Oct 10;40(21):3434-41. doi: 10.1021/jm960810e. J Med Chem. 1997. PMID: 9341918
-
Microbial/enzymatic synthesis of chiral drug intermediates.Adv Appl Microbiol. 2000;47:33-78. doi: 10.1016/s0065-2164(00)47001-2. Adv Appl Microbiol. 2000. PMID: 12876794 Review.
-
Reagents for (ir)reversible enzymatic acylations.Org Biomol Chem. 2003 Jul 21;1(14):2405-15. doi: 10.1039/b302109b. Org Biomol Chem. 2003. PMID: 12956053 Review.
Cited by
-
Cephem-Pyrazinoic Acid Conjugates: Circumventing Resistance in Mycobacterium tuberculosis.Chemistry. 2022 Sep 12;28(51):e202200995. doi: 10.1002/chem.202200995. Epub 2022 Jul 27. Chemistry. 2022. PMID: 35697660 Free PMC article.
-
Synthesis of 7α-Methoxy-7-(4-phenyl-1H-1,2,3-triazol-1-yl)acetamino-3'-arylthio-cephalosporic Acid Derivatives from 7-Aminocephalosporic Acid.Molecules. 2023 Oct 30;28(21):7338. doi: 10.3390/molecules28217338. Molecules. 2023. PMID: 37959756 Free PMC article.
-
Design, syntheses, and anti-tuberculosis activities of conjugates of piperazino-1,3-benzothiazin-4-ones (pBTZs) with 2,7-dimethylimidazo [1,2-a]pyridine-3-carboxylic acids and 7-phenylacetyl cephalosporins.Bioorg Med Chem Lett. 2016 Apr 15;26(8):2068-71. doi: 10.1016/j.bmcl.2016.02.076. Epub 2016 Feb 27. Bioorg Med Chem Lett. 2016. PMID: 26951749 Free PMC article.
-
Antisense Oligonucleotide Activation via Enzymatic Antibiotic Resistance Mechanism.ACS Chem Biol. 2023 Oct 20;18(10):2176-2182. doi: 10.1021/acschembio.3c00027. Epub 2023 Jun 16. ACS Chem Biol. 2023. PMID: 37326511 Free PMC article.
-
Photoinduced meta-Selective C-H Oxygenation of Arenes.JACS Au. 2023 Jun 9;3(6):1790-1799. doi: 10.1021/jacsau.3c00231. eCollection 2023 Jun 26. JACS Au. 2023. PMID: 37388693 Free PMC article.
References
-
- Morin RB, Gorman M, editors. Chemistry and Biology of β-Lactam Antibiotics. Vol. 1 Academic Press; New York: 1982.
-
- Mangia A, Scandroglio A. Org Prep Proced Int. 1986;18:13–15.
- Stedman RJ. J Med Chem. 1966;9:444. - PubMed
-
- Galeazzi R, Martelli G, Mabbili G, Orene M, Rinaldi S. Org Lett. 2004;6:2571–2574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources