Effects of an intensive depression-focused intervention for smoking cessation in pregnancy
- PMID: 20099949
- PMCID: PMC2881321
- DOI: 10.1037/a0018168
Effects of an intensive depression-focused intervention for smoking cessation in pregnancy
Abstract
Objective: The objective of this study was to evaluate a depression-focused treatment for smoking cessation in pregnant women versus a time and contact health education control. We hypothesized that the depression-focused treatment would lead to improved abstinence and reduced depressive symptoms among women with high levels of depressive symptomatology. No significant main effects of treatment were hypothesized.
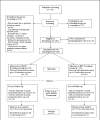
Method: Pregnant smokers (N = 257) were randomly assigned to a 10-week, intensive, depression-focused intervention (cognitive behavioral analysis system of psychotherapy; CBASP) or to a time and contact control focused on health and wellness (HW); both included equivalent amounts of behavioral and motivational smoking cessation counseling. Of the sample, 54% were African American, and 37% met criteria for major depression. Mean age was 25 years (SD = 5.9), and women averaged 19.5 weeks (SD = 8.5) gestation at study entry. We measured symptoms of depression using the Center for Epidemiological Studies-Depression Scale (Radloff, 1977).
Results: At 6 months posttreatment, women with higher levels of baseline depressive symptoms treated with CBASP were abstinent significantly more often, F(1, 253) = 5.61, p = .02, and had less depression, F(1, 2620) = 10.49, p = .001, than those treated with HW; those with low baseline depression fared better in HW. Differences in abstinence were not retained at 6 months postpartum.
Conclusions: The results suggest that pregnant women with high levels of depressive symptoms may benefit from a depression-focused treatment in terms of improved abstinence and depressive symptoms, both of which could have a combined positive effect on maternal and child health.
Figures

References
-
- Adams EK, Melvin CL, Raskind-Hood CL. Sociodemographic, insurance, and risk profiles of maternal smokers post the 1990s: How can we reach them? Nicotine & Tobacco Research. 2008;10:1121–1129. - PubMed
-
- Albrecht SA, Caruthers D, Patrick T, Reynolds M, Salamie D, Higgins LW, Braxter B, Kim Y, Mlynarchek S. A randomized controlled trial of a smoking cessation intervention for pregnant adolescents. Nursing Research. 2006;55:402–410. - PubMed
-
- Alder J, Fink N, Bitzer J, Hosli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. Journal of Maternal-Fetal and Neonatal Medicine. 2007;20:189–209. - PubMed
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Revised ed. American Psychiatric Association; Washington, D.C.: 2000.
-
- Benowitz NL, Ahijevych K, Hall S, Hansson A, Henningfield J, Hurt RD, Jacob PI, Jarvis MJ, LeHouezec J, Lichtenstein E, Tsoh JY, Velicer W. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

