Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering
- PMID: 20100033
- PMCID: PMC2949214
- DOI: 10.1089/ten.TEA.2009.0768
Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering
Abstract
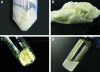
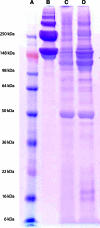
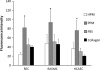
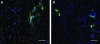
Following ischemic injury in the heart, little to no repair occurs, causing a progressive degeneration of cardiac function that leads to congestive heart failure. Cardiac tissue engineering strategies have focused on designing a variety of injectable scaffolds that range in composition from single-component materials to complex extracellular matrix (ECM)-derived materials. In this study, the pericardial ECM, a commonly used biomaterial, was investigated for use as an injectable scaffold for cardiac repair. It was determined that a solubilized form of decellularized porcine pericardium could be injected and induced to gel in vivo, prompting investigation with human pericardium, which has the decided advantage of offering an autologous therapy. Characterization showed that the matrix gels retained components of the native pericardial ECM, with extant protein and glycosaminoglycan content identified. The results of an in vitro migration assay indicate that the porcine pericardial matrix is a stronger chemoattractant for relevant cell types, but in vivo results showed that the two materials caused statistically similar amounts of neovascularization, demonstrating feasibility as injectable treatments. Potential stem cell mobilization was supported by the presence of c-Kit+ cells within the matrix injection regions. With this work, the pericardium is identified as a novel source for an autologous scaffold for treating myocardial infarction.
Figures




References
-
- Lloyd-Jones D. Adams R. Carnethon M. De Simone G. Ferguson T.B. Flegal K. Ford E. Furie K. Go A. Greenlund K. Haase N. Hailpern S. Ho M. Howard V. Kissela B. Kittner S. Lackland D. Lisabeth L. Marelli A. McDermott M. Meigs J. Mozaffarian D. Nichol G. O'Donnell C. Roger V. Rosamond W. Sacco R. Sorlie P. Stafford R. Steinberger J. Thom T. Wasserthiel-Smoller S. Wong N. Wylie-Rosett J. Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2009 Update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21. - PubMed
-
- Mann D.L. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999;100:999. - PubMed
-
- Laflamme M.A. Murry C.E. Regenerating the heart. Nat Biotechnol. 2005;23:845. - PubMed
-
- Reffelmann T. Kloner R.A. Cellular cardiomyoplasty—cardiomyocytes, skeletal myoblasts, or stem cells for regenerating myocardium and treatment of heart failure? Cardiovasc Res. 2003;58:358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

