Cell-specific targeting of lentiviral vectors mediated by fusion proteins derived from Sindbis virus, vesicular stomatitis virus, or avian sarcoma/leukosis virus
- PMID: 20100344
- PMCID: PMC2823649
- DOI: 10.1186/1742-4690-7-3
Cell-specific targeting of lentiviral vectors mediated by fusion proteins derived from Sindbis virus, vesicular stomatitis virus, or avian sarcoma/leukosis virus
Abstract
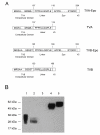
Background: The ability to efficiently and selectively target gene delivery vectors to specific cell types in vitro and in vivo remains one of the formidable challenges in gene therapy. We pursued two different strategies to target lentiviral vector delivery to specific cell types. In one of the strategies, vector particles bearing a membrane-bound stem cell factor sequence plus a separate fusion protein based either on Sindbis virus strain TR339 glycoproteins or the vesicular stomatitis virus G glycoprotein were used to selectively transduce cells expressing the corresponding stem cell factor receptor (c-kit). An alternative approach involved soluble avian sarcoma/leukosis virus receptors fused to cell-specific ligands including stem cell factor and erythropoietin for targeting lentiviral vectors pseudotyped with avian sarcoma/leukosis virus envelope proteins to cells that express the corresponding receptors.
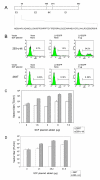
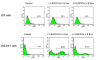
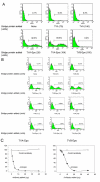
Results: The titers of unconcentrated vector particles bearing Sindbis virus strain TR339 or vesicular stomatitis virus G fusion proteins plus stem cell factor in the context of c-kit expressing cells were up to 3.2 x 10(5) transducing units per ml while vector particles lacking the stem cell factor ligand displayed titers that were approximately 80 fold lower. On cells that lacked the c-kit receptor, the titers of stem cell factor-containing vectors were approximately 40 times lower compared to c-kit-expressing cells. Lentiviral vectors pseudotyped with avian sarcoma/leukosis virus subgroup A or B envelope proteins and bearing bi-functional bridge proteins encoding erythropoietin or stem cell factor fused to the soluble extracellular domains of the avian sarcoma/leukosis virus subgroup A or B receptors resulted in efficient transduction of erythropoietin receptor or c-kit-expressing cells. Transduction of erythropoietin receptor-expressing cells mediated by bi-functional bridge proteins was found to be dependent on the dose, the correct subgroup-specific virus receptor and the correct envelope protein. Furthermore, transduction was completely abolished in the presence of anti-erythropoietin antibody.
Conclusions: Our results indicate that the avian sarcoma/leukosis virus bridge strategy provides a reliable approach for cell-specific lentiviral vector targeting. The background levels were lower compared to alternative strategies involving Sindbis virus strain TR339 or vesicular stomatitis virus fusion proteins.
Figures

Similar articles
-
Cell type-specific targeting with sindbis pseudotyped lentiviral vectors displaying anti-CCR5 single-chain antibodies.Hum Gene Ther. 2005 Feb;16(2):223-34. doi: 10.1089/hum.2005.16.223. Hum Gene Ther. 2005. PMID: 15761262
-
Retargeting Lentiviruses via SpyCatcher-SpyTag Chemistry for Gene Delivery into Specific Cell Types.mBio. 2017 Dec 12;8(6):e01860-17. doi: 10.1128/mBio.01860-17. mBio. 2017. PMID: 29233896 Free PMC article.
-
Engineered lentiviral vectors pseudotyped with a CD4 receptor and a fusogenic protein can target cells expressing HIV-1 envelope proteins.Virus Res. 2011 Sep;160(1-2):340-50. doi: 10.1016/j.virusres.2011.07.010. Epub 2011 Jul 23. Virus Res. 2011. PMID: 21802459 Free PMC article.
-
Pseudotyped Lentiviral Vectors: One Vector, Many Guises.Hum Gene Ther Methods. 2017 Dec;28(6):291-301. doi: 10.1089/hgtb.2017.084. Epub 2017 Sep 4. Hum Gene Ther Methods. 2017. PMID: 28870117 Review.
-
Altering the tropism of lentiviral vectors through pseudotyping.Curr Gene Ther. 2005 Aug;5(4):387-98. doi: 10.2174/1566523054546224. Curr Gene Ther. 2005. PMID: 16101513 Free PMC article. Review.
Cited by
-
Antigen-presenting cell-targeted lentiviral vectors do not support the development of productive T-cell effector responses: implications for in vivo targeted vaccine delivery.Gene Ther. 2017 Jun;24(6):370-375. doi: 10.1038/gt.2017.30. Epub 2017 May 25. Gene Ther. 2017. PMID: 28540936
-
Immunogenicity of targeted lentivectors.Oncotarget. 2014 Feb 15;5(3):704-15. doi: 10.18632/oncotarget.1680. Oncotarget. 2014. PMID: 24519916 Free PMC article.
-
Development of the Nanobody display technology to target lentiviral vectors to antigen-presenting cells.Gene Ther. 2012 Dec;19(12):1133-40. doi: 10.1038/gt.2011.206. Epub 2012 Jan 12. Gene Ther. 2012. PMID: 22241177 Free PMC article.
-
Preferential lentiviral targeting of astrocytes in the central nervous system.PLoS One. 2013 Oct 2;8(10):e76092. doi: 10.1371/journal.pone.0076092. eCollection 2013. PLoS One. 2013. PMID: 24098426 Free PMC article.
-
Lentiviral Vector Bioprocessing.Viruses. 2021 Feb 9;13(2):268. doi: 10.3390/v13020268. Viruses. 2021. PMID: 33572347 Free PMC article. Review.
References
-
- Morizono K, Chen IS. Targeted gene delivery by intravenous injection of retroviral vectors. Cell Cycle. 2005;4:854–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

