Hepatitis C virus infection in blood donors from the state of Puebla, Mexico
- PMID: 20100349
- PMCID: PMC2829021
- DOI: 10.1186/1743-422X-7-18
Hepatitis C virus infection in blood donors from the state of Puebla, Mexico
Abstract
Background: Worldwide, 130 million persons are estimated to be infected with HCV. Puebla is the Mexican state with the highest mortality due to hepatic cirrhosis. Therefore, it is imperative to obtain epidemiological data on HCV infection in asymptomatic people of this region. The objective of present study was to analyze the prevalence of antibodies and genotypes of hepatitis C virus (HCV) in blood donors from Puebla, Mexico.
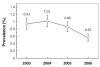
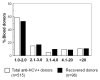
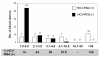
Results: The overall prevalence was 0.84% (515/61553). Distribution by region was: North, 0.86% (54/6270); Southeast, 1.04% (75/7197); Southwest, 0.93% (36/3852); and Central, 0.79% (350/44234). Ninety-six donors were enrolled for detection and genotyping of virus, from which 37 (38.5%) were HCV-RNA positive. Detected subtypes were: 1a (40.5%), 1b (27.0%), mixed 1a/1b (18.9%), undetermined genotype 1 (5.4%), 2a (2.7%), 2b (2.7%), and mixed 1a/2a (2.7%). All recovered donors with S/CO > 39 were HCV-RNA positive (11/11) and presented elevated ALT; in donors with S/CO < 39 HCV-RNA, positivity was of 30.4%; and 70% had normal values of ALT. The main risk factors associated with HCV infection were blood transfusion and surgery.
Conclusions: HCV prevalence of donors in Puebla is similar to other Mexican states. The most prevalent genotype is 1, of which subtype 1a is the most frequent.
Figures

Similar articles
-
Differential prevalence of hepatitis C virus subtypes in healthy blood donors, patients on maintenance hemodialysis, and patients with hepatocellular carcinoma in Surabaya, Indonesia.J Clin Microbiol. 1996 Dec;34(12):2875-80. doi: 10.1128/jcm.34.12.2875-2880.1996. J Clin Microbiol. 1996. PMID: 8940415 Free PMC article.
-
HCV genotypes in Swedish blood donors as correlated to epidemiology, liver disease and hepatitis C virus antibody profile.Infection. 1995 Sep-Oct;23(5):253-7. doi: 10.1007/BF01716280. Infection. 1995. PMID: 8557380
-
Genotyping of hepatitis C virus (HCV) in infected patients from Northeast Mexico.Ann Hepatol. 2008 Apr-Jun;7(2):144-7. Ann Hepatol. 2008. PMID: 18626432
-
Hepatitis C Virus in mainland China with an emphasis on genotype and subtype distribution.Virol J. 2017 Feb 23;14(1):41. doi: 10.1186/s12985-017-0710-z. Virol J. 2017. PMID: 28231805 Free PMC article. Review.
-
Prevalence of HCV genotypes and subtypes in Southeast Asia: A systematic review and meta-analysis.PLoS One. 2021 May 20;16(5):e0251673. doi: 10.1371/journal.pone.0251673. eCollection 2021. PLoS One. 2021. PMID: 34014997 Free PMC article.
Cited by
-
Seroprevalence of anti-HCV antibodies among blood donors of north India.Indian J Med Res. 2013;138(1):125-8. Indian J Med Res. 2013. PMID: 24056566 Free PMC article.
-
Quantitative analysis of interferon alpha receptor subunit 1 and suppressor of cytokine signaling 1 gene transcription in blood cells of patients with chronic hepatitis C.Virol J. 2010 Sep 18;7:243. doi: 10.1186/1743-422X-7-243. Virol J. 2010. PMID: 20849643 Free PMC article.
-
An Update on Viral Hepatitis B and C in Mexico: Advances and Pitfalls in Eradication Strategies.Microorganisms. 2024 Jul 3;12(7):1368. doi: 10.3390/microorganisms12071368. Microorganisms. 2024. PMID: 39065136 Free PMC article. Review.
-
Identification of hepatitis C virus transmission using a next-generation sequencing approach.J Clin Microbiol. 2012 Apr;50(4):1461-3. doi: 10.1128/JCM.00005-12. Epub 2012 Feb 1. J Clin Microbiol. 2012. PMID: 22301026 Free PMC article.
-
Prevalence of hepatitis C in the adult Mexican population: National Survey of Health and Nutrition 2018.Lancet Reg Health Am. 2021 Dec 31;8:100165. doi: 10.1016/j.lana.2021.100165. eCollection 2022 Apr. Lancet Reg Health Am. 2021. PMID: 36778726 Free PMC article.
References
-
- Valdespino JL, Conde-Gonzáles JC, Olaiz FG, Palma O, D K, Sepulveda J. Seroprevalencia de la hepatitis C en adultos de México: ¿un problema de salud pública emergente? Salud Publica Mex. 2007;49:S395–S403.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

