Mutation of the heme axial ligand of Escherichia coli succinate-quinone reductase: implications for heme ligation in mitochondrial complex II from yeast
- PMID: 20100456
- PMCID: PMC2888824
- DOI: 10.1016/j.bbabio.2010.01.019
Mutation of the heme axial ligand of Escherichia coli succinate-quinone reductase: implications for heme ligation in mitochondrial complex II from yeast
Abstract
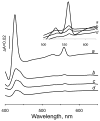
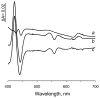
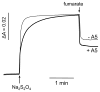
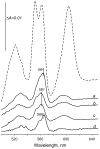
A b-type heme is conserved in membrane-bound complex II enzymes (SQR, succinate-ubiquinone reductase). The axial ligands for the low spin heme b in Escherichia coli complex II are SdhC His84 and SdhD His71. E. coli SdhD His71 is separated by 10 residues from SdhD Asp82 and Tyr83 which are essential for ubiquinone catalysis. The same His-10x-AspTyr motif dominates in homologous SdhD proteins, except for Saccharomyces cerevisiae where a tyrosine is at the axial position (Tyr-Cys-9x-AspTyr). Nevertheless, the yeast enzyme was suggested to contain a stoichiometric amount of heme, however, with the Cys ligand in the aforementioned motif acting as heme ligand. In this report, the role of Cys residues for heme coordination in the complex II family of enzymes is addressed. Cys was substituted to the SdhD-71 position and the yeast Tyr71Cys72 motif was also recreated. The Cys71 variant retained heme, although it was high spin, while the Tyr71Cys72 mutant lacked heme. Previously the presence of heme in S. cerevisiae was detected by a spectral peak in fumarate-oxidized, dithionite-reduced mitochondria. Here it is shown that this method must be used with caution. Comparison of bovine and yeast mitochondrial membranes shows that fumarate induced reoxidation of cytochromes in both SQR and the bc1 complex (ubiquinol-cytochrome c reductase). Thus, this report raises a concern about the presence of low spin heme b in S. cerevisiae complex II.
Published by Elsevier B.V.
Figures
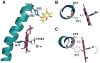

Similar articles
-
Retention of heme in axial ligand mutants of succinate-ubiquinone xxidoreductase (complex II) from Escherichia coli.J Biol Chem. 2001 Jun 1;276(22):18968-76. doi: 10.1074/jbc.M011270200. Epub 2001 Mar 19. J Biol Chem. 2001. PMID: 11259408
-
Localization of histidine residues responsible for heme axial ligation in cytochrome b556 of complex II (succinate:ubiquinone oxidoreductase) in Escherichia coli.Biochemistry. 1998 Mar 24;37(12):4148-59. doi: 10.1021/bi9716635. Biochemistry. 1998. PMID: 9521736
-
Two hydrophobic subunits are essential for the heme b ligation and functional assembly of complex II (succinate-ubiquinone oxidoreductase) from Escherichia coli.J Biol Chem. 1996 Jan 5;271(1):521-7. doi: 10.1074/jbc.271.1.521. J Biol Chem. 1996. PMID: 8550613
-
Wolinella succinogenes quinol:fumarate reductase and its comparison to E. coli succinate:quinone reductase.FEBS Lett. 2003 Nov 27;555(1):21-8. doi: 10.1016/s0014-5793(03)01100-1. FEBS Lett. 2003. PMID: 14630313 Review.
-
Defining a direction: electron transfer and catalysis in Escherichia coli complex II enzymes.Biochim Biophys Acta. 2013 May;1827(5):668-78. doi: 10.1016/j.bbabio.2013.01.010. Epub 2013 Feb 8. Biochim Biophys Acta. 2013. PMID: 23396003 Free PMC article. Review.
Cited by
-
Expression of Saccharomyces cerevisiae Sdh3p and Sdh4p paralogs results in catalytically active succinate dehydrogenase isoenzymes.J Biol Chem. 2012 Jun 29;287(27):22509-20. doi: 10.1074/jbc.M112.344275. Epub 2012 May 9. J Biol Chem. 2012. PMID: 22573324 Free PMC article.
-
Alternative splicing isoform in succinate dehydrogenase complex, subunit C causes downregulation of succinate-coenzyme Q oxidoreductase activity in mitochondria.Oncol Lett. 2015 Jan;9(1):330-334. doi: 10.3892/ol.2014.2699. Epub 2014 Nov 11. Oncol Lett. 2015. PMID: 25435987 Free PMC article.
-
Emerging concepts in the flavinylation of succinate dehydrogenase.Biochim Biophys Acta. 2013 May;1827(5):627-36. doi: 10.1016/j.bbabio.2013.01.012. Epub 2013 Feb 1. Biochim Biophys Acta. 2013. PMID: 23380393 Free PMC article. Review.
-
Mössbauer-based molecular-level decomposition of the Saccharomyces cerevisiae ironome, and preliminary characterization of isolated nuclei.Metallomics. 2022 Nov 1;14(11):mfac080. doi: 10.1093/mtomcs/mfac080. Metallomics. 2022. PMID: 36214417 Free PMC article.
-
From Synthesis to Utilization: The Ins and Outs of Mitochondrial Heme.Cells. 2020 Feb 29;9(3):579. doi: 10.3390/cells9030579. Cells. 2020. PMID: 32121449 Free PMC article. Review.
References
-
- Cecchini G. Function and structure of complex II of the respiratory chain. Annu Rev Biochem. 2003;72:77–109. - PubMed
-
- Hägerhäll C. Succinate:quinone oxidoreductases variation on a conserved theme. Biochim Biophys Acta. 1997;1320:107–141. - PubMed
-
- Cecchini G, Schröder I, Gunsalus RP, Maklashina E. Succinate dehydrogenase and fumarate reductase from Escherichia coli. Biochim Biophys Acta. 2002;1553:140–157. - PubMed
-
- Iverson TM, Luna-Chavez C, Cecchini G, Rees DC. Structure of the Escherichia coli fumarate reductase respiratory complex. Science. 1999;284:1961–1966. - PubMed
-
- Lancaster CR, Kröger A, Auer M, Michel H. Structure of fumarate reductase from Wolinella succinogenes at 2.2 Å resolution. Nature. 1999;402:377–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

