Inhibition of Escherichia coli ATP synthase by amphibian antimicrobial peptides
- PMID: 20100509
- PMCID: PMC2834880
- DOI: 10.1016/j.ijbiomac.2010.01.015
Inhibition of Escherichia coli ATP synthase by amphibian antimicrobial peptides
Abstract
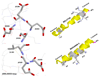
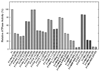
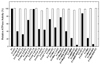
Previously melittin, the alpha-helical basic honey bee venom peptide, was shown to inhibit F(1)-ATPase by binding at the beta-subunit DELSEED motif of F(1)F(o)-ATP synthase. Herein, we present the inhibitory effects of the basic alpha-helical amphibian antimicrobial peptides, ascaphin-8, aurein 2.2, aurein 2.3, carein 1.8, carein 1.9, citropin 1.1, dermaseptin, maculatin 1.1, maganin II, MRP, or XT-7, on purified F(1) and membrane bound F(1)F(0)Escherichia coli ATP synthase. We found that the extent of inhibition by amphibian peptides is variable. Whereas MRP-amide inhibited ATPase essentially completely (approximately 96% inhibition), carein 1.8 did not inhibit at all (0% inhibition). Inhibition by other peptides was partial with a range of approximately 13-70%. MRP-amide was also the most potent inhibitor on molar scale (IC(50) approximately 3.25 microM). Presence of an amide group at the c-terminal of peptides was found to be critical in exerting potent inhibition of ATP synthase ( approximately 20-40% additional inhibition). Inhibition was fully reversible and found to be identical in both F(1)F(0) membrane preparations as well as in isolated purified F(1). Interestingly, growth of E. coli was abrogated in the presence of ascaphin-8, aurein 2.2, aurein 2.3, citropin 1.1, dermaseptin, magainin II-amide, MRP, MRP-amide, melittin, or melittin-amide but was unaffected in the presence of carein 1.8, carein 1.9, maculatin 1.1, magainin II, or XT-7. Hence inhibition of F(1)-ATPase and E. coli cell growth by amphibian antimicrobial peptides suggests that their antimicrobial/anticancer properties are in part linked to their actions on ATP synthase.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Asp residues of βDELSEED-motif are required for peptide binding in the Escherichia coli ATP synthase.Int J Biol Macromol. 2015 Apr;75:37-43. doi: 10.1016/j.ijbiomac.2014.12.047. Epub 2015 Jan 17. Int J Biol Macromol. 2015. PMID: 25603139 Free PMC article.
-
Venom peptides cathelicidin and lycotoxin cause strong inhibition of Escherichia coli ATP synthase.Int J Biol Macromol. 2016 Jun;87:246-51. doi: 10.1016/j.ijbiomac.2016.02.061. Epub 2016 Feb 27. Int J Biol Macromol. 2016. PMID: 26930579 Free PMC article.
-
A connection between antimicrobial properties of venom peptides and microbial ATP synthase.Int J Biol Macromol. 2018 Nov;119:23-31. doi: 10.1016/j.ijbiomac.2018.07.146. Epub 2018 Jul 24. Int J Biol Macromol. 2018. PMID: 30053390
-
Medicinal chemistry of ATP synthase: a potential drug target of dietary polyphenols and amphibian antimicrobial peptides.Curr Med Chem. 2010;17(25):2822-36. doi: 10.2174/092986710791859270. Curr Med Chem. 2010. PMID: 20586714 Free PMC article. Review.
-
Membrane interactions of antimicrobial peptides from Australian frogs.Biochim Biophys Acta. 2009 Aug;1788(8):1630-8. doi: 10.1016/j.bbamem.2008.10.007. Epub 2008 Oct 25. Biochim Biophys Acta. 2009. PMID: 19013126 Review.
Cited by
-
Asp residues of βDELSEED-motif are required for peptide binding in the Escherichia coli ATP synthase.Int J Biol Macromol. 2015 Apr;75:37-43. doi: 10.1016/j.ijbiomac.2014.12.047. Epub 2015 Jan 17. Int J Biol Macromol. 2015. PMID: 25603139 Free PMC article.
-
Dermaseptins, Multifunctional Antimicrobial Peptides: A Review of Their Pharmacology, Effectivity, Mechanism of Action, and Possible Future Directions.Front Pharmacol. 2019 Nov 26;10:1421. doi: 10.3389/fphar.2019.01421. eCollection 2019. Front Pharmacol. 2019. PMID: 31849670 Free PMC article. Review.
-
Improving Phylogeny Reconstruction at the Strain Level Using Peptidome Datasets.PLoS Comput Biol. 2016 Dec 29;12(12):e1005271. doi: 10.1371/journal.pcbi.1005271. eCollection 2016 Dec. PLoS Comput Biol. 2016. PMID: 28033346 Free PMC article.
-
Potential Inhibitory Effect of Apis mellifera's Venom and of Its Two Main Components-Melittin and PLA2-on Escherichia coli F1F0-ATPase.Antibiotics (Basel). 2020 Nov 18;9(11):824. doi: 10.3390/antibiotics9110824. Antibiotics (Basel). 2020. PMID: 33218209 Free PMC article.
-
Spotlight on the Selected New Antimicrobial Innate Immune Peptides Discovered During 2015-2019.Curr Top Med Chem. 2020;20(32):2984-2998. doi: 10.2174/1568026620666201022143625. Curr Top Med Chem. 2020. PMID: 33092508 Free PMC article. Review.
References
-
- Abrahams JP, Leslie AGW, Lutter R, Walker JE. Nature. 1994;370:621–628. - PubMed
-
- Senior AE, Nadanaciva S, Weber J. Biochim Biophys Acta. 2002;1553:188–211. - PubMed
-
- Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Nature. 1997;386:299–302. - PubMed
-
- Diez M, Zimmermann B, Borsch M, Konig M, Schweinberger E, Steigmiller S, Reuter R, Felekyan S, Kudryavtsev V, Seidel CAM, Graber P. Nat Struct Mol Biol. 2004;11:135–141. - PubMed
-
- Itoh H, Takahashi A, Adachi K, Noji H, Yasuda R, Yoshida M, Kinosita K. Nature. 2004;427:465–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

