Viruses as vesicular carriers of the viral genome: a functional module perspective
- PMID: 20100522
- PMCID: PMC7114299
- DOI: 10.1016/j.bbamcr.2010.01.011
Viruses as vesicular carriers of the viral genome: a functional module perspective
Abstract
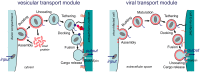
Enveloped viruses and cellular transport vesicles share obvious morphological and functional properties. Both are composed of a closed membrane, which is lined with coat proteins and encases cargo. Transmembrane proteins inserted into the membrane define the target membrane area with which the vesicle or virus is destined to fuse. Here we discuss recent insight into the functioning of enveloped viruses in the framework of the "functional module" concept. Vesicular transport is an exemplary case of a functional module, as defined as a part of the proteome that assembles to perform a specific autonomous function in a living cell. Cellular vesicles serve to transport cargo between membranous organelles inside the cell, while enveloped viruses can be seen as carriers of the viral genome delivering their cargo from an infected to an uninfected cell. The turnover of both vesicles and viruses involves an analogous series of submodular events. This comprises assembly of elements, budding from the donor compartment, uncoating and/or maturation, docking to and finally fusion with the target membrane to release the cargo. This modular perception enables us to define submodular building blocks so that mechanisms and elements can be directly compared. It will be analyzed where viruses have developed their own specific strategy, where they share functional schemes with vesicles, and also where they even have "hijacked" complete submodular schemes from the cell. Such a perspective may also include new and more specific approaches to pharmacological interference with virus function, which could avoid some of the most severe side effects.
2010 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Vesicular transport.Essays Biochem. 2000;36:37-46. doi: 10.1042/bse0360037. Essays Biochem. 2000. PMID: 12471901 Review.
-
Generation of nonidentical compartments in vesicular transport systems.J Cell Biol. 2005 Jan 17;168(2):271-80. doi: 10.1083/jcb.200409087. J Cell Biol. 2005. PMID: 15657397 Free PMC article.
-
Structure of membrane tethers and their role in fusion.Traffic. 2019 Jul;20(7):479-490. doi: 10.1111/tra.12655. Epub 2019 May 30. Traffic. 2019. PMID: 31062920 Review.
-
Distinct stages in the recognition, sorting, and packaging of proTGFα into COPII-coated transport vesicles.Mol Biol Cell. 2016 Jun 15;27(12):1938-47. doi: 10.1091/mbc.E16-02-0090. Epub 2016 Apr 27. Mol Biol Cell. 2016. PMID: 27122606 Free PMC article.
-
A complete set of SNAREs in yeast.Traffic. 2004 Jan;5(1):45-52. doi: 10.1046/j.1600-0854.2003.00151.x. Traffic. 2004. PMID: 14675424
Cited by
-
Association of influenza virus proteins with membrane rafts.Adv Virol. 2011;2011:370606. doi: 10.1155/2011/370606. Epub 2011 Jul 25. Adv Virol. 2011. PMID: 22312341 Free PMC article.
-
Membrane proteins of arterivirus particles: structure, topology, processing and function.Virus Res. 2014 Dec 19;194:16-36. doi: 10.1016/j.virusres.2014.09.010. Epub 2014 Sep 30. Virus Res. 2014. PMID: 25278143 Free PMC article. Review.
-
Lipids and pathogenic flaviviruses: An intimate union.PLoS Pathog. 2018 May 10;14(5):e1006952. doi: 10.1371/journal.ppat.1006952. eCollection 2018 May. PLoS Pathog. 2018. PMID: 29746606 Free PMC article. No abstract available.
-
Common Nodes of Virus-Host Interaction Revealed Through an Integrated Network Analysis.Front Immunol. 2019 Oct 4;10:2186. doi: 10.3389/fimmu.2019.02186. eCollection 2019. Front Immunol. 2019. PMID: 31636628 Free PMC article.
-
Structure of the MxA stalk elucidates the assembly of ring-like units of an antiviral module.Small GTPases. 2010 Jul;1(1):62-64. doi: 10.4161/sgtp.1.1.12989. Small GTPases. 2010. PMID: 21686120 Free PMC article.
References
-
- Hartwell L.H., Hopfield J.J., Leibler S., Murray A.W. From molecular to modular cell biology. Nature. 1999;402:C47–C52. - PubMed
-
- Hofmann K.P., Spahn C.M., Heinrich R., Heinemann U. Building functional modules from molecular interactions. Trends Biochem. Sci. 2006;31:497–508. - PubMed
-
- Kummel D., Heinemann U. Diversity in structure and function of tethering complexes: evidence for different mechanisms in vesicular transport regulation. Curr. Protein Pept. Sci. 2008;9:197–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

