Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein
- PMID: 20100527
- PMCID: PMC2832096
- DOI: 10.1016/j.jbiotec.2010.01.011
Selective targeting of a TNFR decoy receptor pharmaceutical to the primate brain as a receptor-specific IgG fusion protein
Abstract
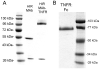
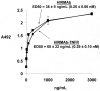
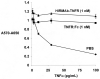
Decoy receptors, such as the human tumor necrosis factor receptor (TNFR), are potential new therapies for brain disorders. However, decoy receptors are large molecule drugs that are not transported across the blood-brain barrier (BBB). To enable BBB transport of a TNFR decoy receptor, the human TNFR-II extracellular domain was re-engineered as a fusion protein with a chimeric monoclonal antibody (MAb) against the human insulin receptor (HIR). The HIRMAb acts as a molecular Trojan horse to ferry the TNFR therapeutic decoy receptor across the BBB. The HIRMAb-TNFR fusion protein was expressed in stably transfected CHO cells, and was analyzed with electrophoresis, Western blotting, size exclusion chromatography, and binding assays for the HIR and TNFalpha. The HIRMAb-TNFR fusion protein was radio-labeled by trititation, in parallel with the radio-iodination of recombinant TNFR:Fc fusion protein, and the proteins were co-injected in the adult Rhesus monkey. The TNFR:Fc fusion protein did not cross the primate BBB in vivo, but the uptake of the HIRMAb-TNFR fusion protein was high and 3% of the injected dose was taken up by the primate brain. The TNFR was selectively targeted to brain, relative to peripheral organs, following fusion to the HIRMAb. This study demonstrates that decoy receptors may be re-engineered as IgG fusion proteins with a BBB molecular Trojan horse that selectively targets the brain, and enables penetration of the BBB in vivo. IgG-decoy receptor fusion proteins represent a new class of human neurotherapeutics.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures


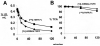


Similar articles
-
Biologic TNFα-inhibitors that cross the human blood-brain barrier.Bioeng Bugs. 2010 Jul-Aug;1(4):231-4. doi: 10.4161/bbug.1.4.12105. Epub 2010 Apr 14. Bioeng Bugs. 2010. PMID: 21327054 Free PMC article.
-
Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier.Methods Enzymol. 2012;503:269-92. doi: 10.1016/B978-0-12-396962-0.00011-2. Methods Enzymol. 2012. PMID: 22230573 Review.
-
Drug targeting of erythropoietin across the primate blood-brain barrier with an IgG molecular Trojan horse.J Pharmacol Exp Ther. 2010 Jun;333(3):961-9. doi: 10.1124/jpet.109.165092. Epub 2010 Mar 16. J Pharmacol Exp Ther. 2010. PMID: 20233799
-
Pharmacokinetics and brain uptake in the rhesus monkey of a fusion protein of arylsulfatase a and a monoclonal antibody against the human insulin receptor.Biotechnol Bioeng. 2013 May;110(5):1456-65. doi: 10.1002/bit.24795. Epub 2012 Dec 25. Biotechnol Bioeng. 2013. PMID: 23192358 Free PMC article.
-
Delivery of Biologics Across the Blood-Brain Barrier with Molecular Trojan Horse Technology.BioDrugs. 2017 Dec;31(6):503-519. doi: 10.1007/s40259-017-0248-z. BioDrugs. 2017. PMID: 29067674 Review.
Cited by
-
Pharmacokinetics and brain uptake of an IgG-TNF decoy receptor fusion protein following intravenous, intraperitoneal, and subcutaneous administration in mice.Mol Pharm. 2013 Apr 1;10(4):1425-31. doi: 10.1021/mp400004a. Epub 2013 Feb 28. Mol Pharm. 2013. PMID: 23410508 Free PMC article.
-
Cyanidin prevents MDPV withdrawal-induced anxiety-like effects and dysregulation of cytokine systems in rats.Brain Res. 2023 May 1;1806:148310. doi: 10.1016/j.brainres.2023.148310. Epub 2023 Mar 4. Brain Res. 2023. PMID: 36871847 Free PMC article.
-
A Historical Review of Brain Drug Delivery.Pharmaceutics. 2022 Jun 16;14(6):1283. doi: 10.3390/pharmaceutics14061283. Pharmaceutics. 2022. PMID: 35745855 Free PMC article. Review.
-
Therapeutic inhibition of soluble brain TNF promotes remyelination by increasing myelin phagocytosis by microglia.JCI Insight. 2017 Apr 20;2(8):e87455. doi: 10.1172/jci.insight.87455. eCollection 2017 Apr 20. JCI Insight. 2017. PMID: 28422748 Free PMC article.
-
Agile delivery of protein therapeutics to CNS.J Control Release. 2014 Sep 28;190:637-63. doi: 10.1016/j.jconrel.2014.06.017. Epub 2014 Jun 21. J Control Release. 2014. PMID: 24956489 Free PMC article. Review.
References
-
- Boado RJ, Zhang Y, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood-brain barrier. Biotechnol Bioeng. 2007a;96:381–391. - PubMed
-
- Boado RJ, Zhang Y, Zhang Y, Pardridge WM. Genetic engineering, expression, and activity of a fusion protein of a human neurotrophin and a molecular Trojan horse for delivery across the human blood-brain barrier. Biotechnol Bioeng. 2007b;97:1376–1386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

