Novel aspects of corneal angiogenic and lymphangiogenic privilege
- PMID: 20100589
- PMCID: PMC3685179
- DOI: 10.1016/j.preteyeres.2010.01.002
Novel aspects of corneal angiogenic and lymphangiogenic privilege
Abstract
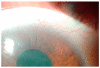
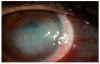
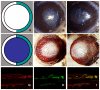
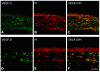
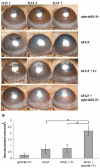
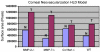
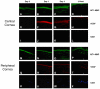
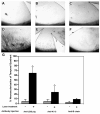
In this article, we provide the results of experimental studies demonstrating that corneal avascularity is an active process involving the production of anti-angiogenic factors, which counterbalance the pro-angiogenic/lymphangiogenic factors that are upregulated during wound healing. We also summarize pertinent published reports regarding corneal neovascularization (NV), corneal lymphangiogenesis and corneal angiogenic/lymphangiogenic privilege. We outline the clinical causes of corneal NV, and discuss the angiogenic proteins (VEGF and bFGF) and angiogenesis regulatory proteins. We also describe the role of matrix metalloproteinases MMP-2, -7, and MT1-MMP, anti-angiogenic factors, and lymphangiogenic regulatory proteins during corneal wound healing. Established and potential new therapies for the treatment of corneal neovascularization are also discussed.
(c) 2010. Published by Elsevier Ltd.
Figures














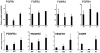






References
-
- Abbas A, Khan B, Feroze AH, Hyman GF. Thalidomide prevents donor corneal graft neovascularization in an alkali burn model of corneal angiogenesis. J. Pak. Med. Assoc. 2002;52:476–482. - PubMed
-
- Abdiu O, Van Setten G. Antiangiogenic activity in tears: presence of pigment-epithelium-derived factor. New insights and preliminary results. Ophthalmic Res. 2008;40:16–18. - PubMed
-
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J. Biol. Chem. 1997;272:15442–15451. - PubMed
-
- Acuff HB, Sinnamon M, Fingleton B, Boone B, Levy SE, Chen X, Pozzi A, Carbone DP, Schwartz DR, Moin K, Sloane BF, Matrisian LM. Analysis of host- and tumor-derived proteinases using a custom dual species microarray reveals a protective role for stromal matrix metalloproteinase-12 in non-small cell lung cancer. Cancer Res. 2006;66:7968–7975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

