Importance of membrane structural integrity for RPE65 retinoid isomerization activity
- PMID: 20100834
- PMCID: PMC2843217
- DOI: 10.1074/jbc.M109.063941
Importance of membrane structural integrity for RPE65 retinoid isomerization activity
Abstract
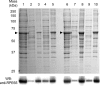
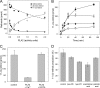
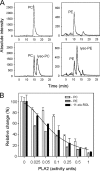
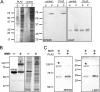
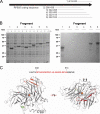
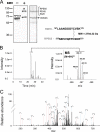
Regeneration of visual chromophore in the vertebrate visual cycle involves the retinal pigment epithelium-specific protein RPE65, the key enzyme catalyzing the cleavage and isomerization of all-trans-retinyl fatty acid esters to 11-cis-retinol. Although RPE65 has no predicted membrane spanning domains, this protein predominantly associates with microsomal fractions isolated from bovine retinal pigment epithelium (RPE). We have re-examined the nature of RPE65 interactions with native microsomal membranes by using extraction and phase separation experiments. We observe that hydrophobic interactions are the dominant forces that promote RPE65 association with these membranes. These results are consistent with the crystallographic model of RPE65, which features a large lipophilic surface that surrounds the entrance to the catalytic site of this enzyme and likely interacts with the hydrophobic core of the endoplasmic reticulum membrane. Moreover, we report a critical role for phospholipid membranes in preserving the retinoid isomerization activity and physical properties of RPE65. Isomerase activity measured in bovine RPE was highly sensitive to phospholipase A(2) treatment, but the observed decline in 11-cis-retinol production did not directly reflect inhibition by products of lipid hydrolysis. Instead, a direct correlation between the kinetics of phospholipid hydrolysis and retinoid isomerization suggests that the lipid membrane structure is critical for RPE65 enzymatic activity. We also provide evidence that RPE65 operates in a multiprotein complex with retinol dehydrogenase 5 and retinal G protein-coupled receptor in RPE microsomes. Modifications in the phospholipid environment affecting interactions with these protein components may be responsible for the alterations in retinoid metabolism observed in phospholipid-depleted RPE microsomes. Thus, our results indicate that the enzymatic activity of native RPE65 strongly depends on its membrane binding and phospholipid environment.
Figures

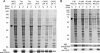

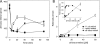


Similar articles
-
Crystal structure of native RPE65, the retinoid isomerase of the visual cycle.Proc Natl Acad Sci U S A. 2009 Oct 13;106(41):17325-30. doi: 10.1073/pnas.0906600106. Epub 2009 Oct 5. Proc Natl Acad Sci U S A. 2009. PMID: 19805034 Free PMC article.
-
Analysis of the retinoid isomerase activities in the retinal pigment epithelium and retina.Methods Mol Biol. 2010;652:329-39. doi: 10.1007/978-1-60327-325-1_19. Methods Mol Biol. 2010. PMID: 20552438 Free PMC article.
-
Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells.J Biol Chem. 2004 Jan 2;279(1):635-43. doi: 10.1074/jbc.M310042200. Epub 2003 Oct 7. J Biol Chem. 2004. PMID: 14532273
-
RPE65 Palmitoylation: A Tale of Lipid Posttranslational Modification.Adv Exp Med Biol. 2019;1185:537-541. doi: 10.1007/978-3-030-27378-1_88. Adv Exp Med Biol. 2019. PMID: 31884667 Free PMC article. Review.
-
Membrane-binding and enzymatic properties of RPE65.Prog Retin Eye Res. 2010 Sep;29(5):428-42. doi: 10.1016/j.preteyeres.2010.03.002. Epub 2010 Mar 19. Prog Retin Eye Res. 2010. PMID: 20304090 Free PMC article. Review.
Cited by
-
LRAT-specific domain facilitates vitamin A metabolism by domain swapping in HRASLS3.Nat Chem Biol. 2015 Jan;11(1):26-32. doi: 10.1038/nchembio.1687. Epub 2014 Nov 10. Nat Chem Biol. 2015. PMID: 25383759 Free PMC article.
-
Structure and mechanism of NOV1, a resveratrol-cleaving dioxygenase.Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):14324-14329. doi: 10.1073/pnas.1608917113. Epub 2016 Nov 30. Proc Natl Acad Sci U S A. 2016. PMID: 27911781 Free PMC article.
-
Safer and efficient base editing and prime editing via ribonucleoproteins delivered through optimized lipid-nanoparticle formulations.Nat Biomed Eng. 2025 Jan;9(1):57-78. doi: 10.1038/s41551-024-01296-2. Epub 2024 Nov 28. Nat Biomed Eng. 2025. PMID: 39609561 Free PMC article.
-
The Usher 1B protein, MYO7A, is required for normal localization and function of the visual retinoid cycle enzyme, RPE65.Hum Mol Genet. 2011 Jul 1;20(13):2560-70. doi: 10.1093/hmg/ddr155. Epub 2011 Apr 14. Hum Mol Genet. 2011. PMID: 21493626 Free PMC article.
-
Temperature-sensitive retinoid isomerase activity of RPE65 mutants associated with Leber Congenital Amaurosis.J Biochem. 2015 Aug;158(2):115-25. doi: 10.1093/jb/mvv028. Epub 2015 Mar 9. J Biochem. 2015. PMID: 25752820 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

