Mutation of actin Tyr-53 alters the conformations of the DNase I-binding loop and the nucleotide-binding cleft
- PMID: 20100837
- PMCID: PMC2843222
- DOI: 10.1074/jbc.M109.073452
Mutation of actin Tyr-53 alters the conformations of the DNase I-binding loop and the nucleotide-binding cleft
Abstract
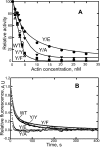
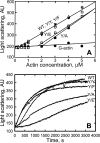
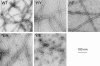
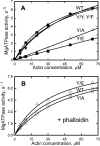
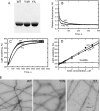
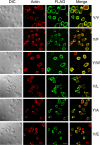
All but 11 of the 323 known actin sequences have Tyr at position 53, and the 11 exceptions have the conservative substitution Phe, which raises the following questions. What is the critical role(s) of Tyr-53, and, if it can be replaced by Phe, why has this happened so infrequently? We compared the properties of purified endogenous Dictyostelium actin and mutant constructs with Tyr-53 replaced by Phe, Ala, Glu, Trp, and Leu. The Y53F mutant did not differ significantly from endogenous actin in any of the properties assayed, but the Y53A and Y53E mutants differed substantially; affinity for DNase I was reduced, the rate of nucleotide exchange was increased, the critical concentration for polymerization was increased, filament elongation was inhibited, and polymerized actin was in the form of small oligomers and imperfect filaments. Growth and/or development of cells expressing these actin mutants were also inhibited. The Trp and Leu mutations had lesser but still significant effects on cell phenotype and the biochemical properties of the purified actins. We conclude that either Tyr or Phe is required to maintain the functional conformations of the DNase I-binding loop (D-loop) in both G- and F-actin, and that the conformation of the D-loop affects not only the properties that directly involve the D-loop (binding to DNase I and polymerization) but also allosterically modifies the conformation of the nucleotide-binding cleft, thus increasing the rate of nucleotide exchange. The apparent evolutionary "preference" for Tyr at position 53 may be the result of Tyr allowing dynamic modification of the D-loop conformation by phosphorylation (Baek, K., Liu, X., Ferron, F., Shu, S., Korn, E. D., and Dominguez, R. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11748-11753) with effects similar, but not identical, to those of the Ala and Glu mutations.
Figures


Similar articles
-
Expression of Y53A-actin in Dictyostelium disrupts the cytoskeleton and inhibits intracellular and intercellular chemotactic signaling.J Biol Chem. 2010 Sep 3;285(36):27713-25. doi: 10.1074/jbc.M110.116277. Epub 2010 Jul 7. J Biol Chem. 2010. PMID: 20610381 Free PMC article.
-
Modulation of actin structure and function by phosphorylation of Tyr-53 and profilin binding.Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11748-53. doi: 10.1073/pnas.0805852105. Epub 2008 Aug 8. Proc Natl Acad Sci U S A. 2008. PMID: 18689676 Free PMC article.
-
Amino acids 519-524 of Dictyostelium myosin II form a surface loop that aids actin binding by facilitating a conformational change.J Muscle Res Cell Motil. 2002;23(7-8):685-95. doi: 10.1023/a:1024463325335. J Muscle Res Cell Motil. 2002. PMID: 12952067
-
Dictyostelium myosin II as a model to study the actin-myosin interactions during force generation.J Muscle Res Cell Motil. 2002;23(7-8):697-702. doi: 10.1023/a:1024415409406. J Muscle Res Cell Motil. 2002. PMID: 12952068 Review.
-
Two conformations of G-actin related to two conformations of F-actin.Results Probl Cell Differ. 2001;32:95-101. doi: 10.1007/978-3-540-46560-7_7. Results Probl Cell Differ. 2001. PMID: 11131839 Review.
Cited by
-
Biochemical and biological properties of cortexillin III, a component of Dictyostelium DGAP1-cortexillin complexes.Mol Biol Cell. 2014 Jul 1;25(13):2026-38. doi: 10.1091/mbc.E13-08-0457. Epub 2014 May 7. Mol Biol Cell. 2014. PMID: 24807902 Free PMC article.
-
Expression of Y53A-actin in Dictyostelium disrupts the cytoskeleton and inhibits intracellular and intercellular chemotactic signaling.J Biol Chem. 2010 Sep 3;285(36):27713-25. doi: 10.1074/jbc.M110.116277. Epub 2010 Jul 7. J Biol Chem. 2010. PMID: 20610381 Free PMC article.
-
Oxidative hotspots on actin promote skeletal muscle weakness in rheumatoid arthritis.JCI Insight. 2019 Mar 28;5(9):e126347. doi: 10.1172/jci.insight.126347. JCI Insight. 2019. PMID: 30920392 Free PMC article.
-
Actin cross-linking proteins cortexillin I and II are required for cAMP signaling during Dictyostelium chemotaxis and development.Mol Biol Cell. 2012 Jan;23(2):390-400. doi: 10.1091/mbc.E11-09-0764. Epub 2011 Nov 23. Mol Biol Cell. 2012. PMID: 22114350 Free PMC article.
-
JMJD6 regulates histone H2A.X phosphorylation and promotes autophagy in triple-negative breast cancer cells via a novel tyrosine kinase activity.Oncogene. 2019 Feb;38(7):980-997. doi: 10.1038/s41388-018-0466-y. Epub 2018 Sep 5. Oncogene. 2019. PMID: 30185813
References
-
- Sheterline P., Clayton J., Sparrow J. C. (1998) Protein Profile Actin, 4th Ed., p. 44, Oxford University Press, Oxford
-
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. (1990) Nature 347, 37–44 - PubMed
-
- Schweiger A., Mihalache O., Ecke M., Gerisch G. (1992) J. Cell Sci. 102, 601–609 - PubMed
-
- Jungbluth A., von Arnim V., Biegelmann E., Humbel B., Schweiger A., Gerisch G. (1994) J. Cell Sci. 107, 117–125 - PubMed
-
- Jungbluth A., Eckerskorn C., Gerisch G., Lottspeich F., Stocker S., Schweiger A. (1995) FEBS Lett. 375, 87–90 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

