Promyelocytic leukemia protein controls cell migration in response to hydrogen peroxide and insulin-like growth factor-1
- PMID: 20100838
- PMCID: PMC2843199
- DOI: 10.1074/jbc.M109.063362
Promyelocytic leukemia protein controls cell migration in response to hydrogen peroxide and insulin-like growth factor-1
Abstract
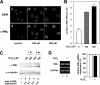
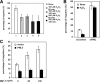
Promyelocytic leukemia protein (PML) was originally identified as part of a chromosomal translocation that contributes to the development of acute promyelocytic leukemia (APL). Since its discovery, PML has been found to play diverse roles in different cellular processes. Notably, PML has anti-proliferative and pro-apoptotic activity that supports its role as a tumor suppressor. We have previously shown that the peptidyl-prolyl isomerase Pin1 is able to affect cell proliferation and hydrogen peroxide (H(2)O(2))-mediated cell death through modulation of the steady-state levels of PML. We have extended these studies to show that the interaction between PML and Pin1 is targeted by multiple extracellular signals in the cell. We show that H(2)O(2) up-regulates and IGF-1 down-regulates PML expression in a Pin1-dependent manner. Interestingly, we found that H(2)O(2)- and IGF-1-mediated alteration in PML accumulation regulate MDA-MB-231 cell migration. Furthermore, we show that the control of cell migration by PML, and thus H(2)O(2) and IGF-1, results from PML-dependent decreased expression of integrin beta1 (ITGB1). Knockdown of Pin1 leads to decreased cell migration, lower levels of ITGB1 expression and resistance to IGF-1- and H(2)O(2)-induced changes in cell migration and ITGB1 expression. Taken together, our work identifies PML as a common target for H(2)O(2) and IGF-1 and supports a novel tumor suppressive role for PML in controlling cell migration through the expression of ITGB1.
Figures
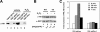





Similar articles
-
Degradation of the tumor suppressor PML by Pin1 contributes to the cancer phenotype of breast cancer MDA-MB-231 cells.Mol Cell Biol. 2008 Feb;28(3):997-1006. doi: 10.1128/MCB.01848-07. Epub 2007 Nov 26. Mol Cell Biol. 2008. PMID: 18039859 Free PMC article.
-
Mitogen-activated protein kinase extracellular signal-regulated kinase 2 phosphorylates and promotes Pin1 protein-dependent promyelocytic leukemia protein turnover.J Biol Chem. 2011 Dec 30;286(52):44403-11. doi: 10.1074/jbc.M111.289512. Epub 2011 Oct 27. J Biol Chem. 2011. PMID: 22033920 Free PMC article.
-
Active Pin1 is a key target of all-trans retinoic acid in acute promyelocytic leukemia and breast cancer.Nat Med. 2015 May;21(5):457-66. doi: 10.1038/nm.3839. Epub 2015 Apr 13. Nat Med. 2015. PMID: 25849135 Free PMC article.
-
Promyelocytic leukemia protein (PML) regulates endothelial cell network formation and migration in response to tumor necrosis factor α (TNFα) and interferon α (IFNα).J Biol Chem. 2012 Jul 6;287(28):23356-67. doi: 10.1074/jbc.M112.340505. Epub 2012 May 15. J Biol Chem. 2012. PMID: 22589541 Free PMC article.
-
Therapy-induced PML/RARA proteolysis and acute promyelocytic leukemia cure.Clin Cancer Res. 2009 Oct 15;15(20):6321-6. doi: 10.1158/1078-0432.CCR-09-0209. Epub 2009 Oct 6. Clin Cancer Res. 2009. PMID: 19808868 Review.
Cited by
-
New Strategies to Direct Therapeutic Targeting of PML to Treat Cancers.Front Oncol. 2013 May 17;3:124. doi: 10.3389/fonc.2013.00124. eCollection 2013. Front Oncol. 2013. PMID: 23730625 Free PMC article.
-
PML Degradation: Multiple Ways to Eliminate PML.Front Oncol. 2013 Mar 22;3:60. doi: 10.3389/fonc.2013.00060. eCollection 2013. Front Oncol. 2013. PMID: 23526763 Free PMC article.
-
Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy.Oncotarget. 2017 Jul 25;8(41):71249-71284. doi: 10.18632/oncotarget.19531. eCollection 2017 Sep 19. Oncotarget. 2017. PMID: 29050360 Free PMC article. Review.
-
Mechanism of Notch Signaling Pathway in Malignant Progression of Glioblastoma and Targeted Therapy.Biomolecules. 2024 Apr 15;14(4):480. doi: 10.3390/biom14040480. Biomolecules. 2024. PMID: 38672496 Free PMC article. Review.
-
USP11 regulates PML stability to control Notch-induced malignancy in brain tumours.Nat Commun. 2014;5:3214. doi: 10.1038/ncomms4214. Nat Commun. 2014. PMID: 24487962 Free PMC article.
References
-
- Melnick A., Licht J. D. (1999) Blood 93, 3167–3215 - PubMed
-
- Maul G. G., Negorev D., Bell P., Ishov A. M. (2000) J. Struct. Biol. 129, 278–287 - PubMed
-
- Ching R. W., Dellaire G., Eskiw C. H., Bazett-Jones D. P. (2005) J. Cell Sci. 118, 847–854 - PubMed
-
- Gurrieri C., Capodieci P., Bernardi R., Scaglioni P. P., Nafa K., Rush L. J., Verbel D. A., Cordon-Cardo C., Pandolfi P. P. (2004) J. Natl. Cancer Inst. 96, 269–279 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

