Biochemical and biological characterization of a new oxidized avidin with enhanced tissue binding properties
- PMID: 20100839
- PMCID: PMC2838329
- DOI: 10.1074/jbc.M109.080457
Biochemical and biological characterization of a new oxidized avidin with enhanced tissue binding properties
Abstract
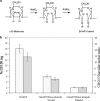
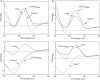
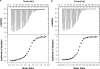
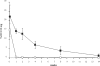
Chicken avidin and bacterial streptavidin are widely employed in vitro for their capacity to bind biotin, but their pharmacokinetics and immunological properties are not always optimal, thereby limiting their use in medical treatments. Here we investigate the biochemical and biological properties of a new modified avidin, obtained by ligand-assisted sodium periodate oxidation of avidin. This method allows protection of biotin-binding sites of avidin from inactivation caused by the oxidation step and delay of avidin clearance from injected tissue by generation of aldehyde groups from avidin carbohydrate moieties. Oxidized avidin shows spectroscopic properties similar to that of native avidin, indicating that tryptophan residues are spared from oxidation damage. In strict agreement with these results, circular dichroism and isothermal titration calorimetry analyses confirm that the ligand-assisted oxidation preserves the avidin protein structure and its biotin binding capacity. In vitro cell binding and in vivo tissue residence experiments demonstrate that aldehyde groups provide oxidized avidin the property to bind cellular and interstitial protein amino groups through Schiff's base formation, resulting in a tissue half-life of 2 weeks, compared with 2 h of native avidin. In addition, the efficient uptake of the intravenously injected (111)In-BiotinDOTA (ST2210) in the site previously treated with modified avidin underlines that tissue-bound oxidized avidin retains its biotin binding capacity in vivo. The results presented here indicate that oxidized avidin could be employed to create a stable artificial receptor in diseased tissues for the targeting of biotinylated therapeutics.
Figures


References
-
- Green N. M. (1990) Methods Enzymol. 184, 51–67 - PubMed
-
- Wilchek M., Bayer E. A. (1990) Methods Enzymol. 184, 5–13 - PubMed
-
- Hnatowich D. J., Virzi F., Rusckowski M. (1987) J. Nucl. Med. 28, 1294–1302 - PubMed
-
- Sakahara H., Saga T. (1999) Adv. Drug Deliv. Rev. 37, 89–101 - PubMed
-
- Oehr P., Westermann J., Biersack H. J. (1988) J. Nucl. Med. 29, 728–729 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

