Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to Nontyphoidal Salmonella enterica serovar typhimurium infection in mice
- PMID: 20100860
- PMCID: PMC2849399
- DOI: 10.1128/IAI.00887-09
Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to Nontyphoidal Salmonella enterica serovar typhimurium infection in mice
Abstract
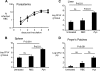
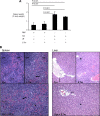
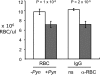
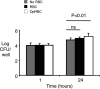
Severe pediatric malaria is an important risk factor for developing disseminated infections with nontyphoidal Salmonella serotypes (NTS). While recent animal studies on this subject are lacking, early work suggests that an increased risk for developing systemic NTS infection during malaria is caused by hemolytic anemia, which leads to reduced macrophage microbicidal activity. Here we established a model for oral Salmonella enterica serotype Typhimurium challenge in mice infected with Plasmodium yoelii nigeriensis. Initial characterization of this model showed that 5 days after coinoculation, P. yoelii nigeriensis infection increased the recovery of S. Typhimurium from liver and spleen by approximately 1,000-fold. The increased bacterial burden could be only partially recapitulated by antibody-mediated hemolysis, which increased the recovery of S. Typhimurium from liver and spleen by 10-fold. These data suggested that both hemolysis and P. yoelii nigeriensis-specific factors contributed to the increased susceptibility to S. Typhimurium. The mechanism by which hemolysis impaired resistance to S. Typhimurium was further investigated. In vitro, S. Typhimurium was recovered 24 h after infection of hemophagocytic macrophages in 2-fold-higher numbers than after infection of mock-treated macrophages, making it unlikely that reduced macrophage microbicidal activity was solely responsible for hemolysis-induced immunosuppression during malaria. Infection with P. yoelii nigeriensis, but not antibody-mediated hemolysis, reduced serum levels of interleukin-12p70 (IL-12p70) in response to S. Typhimurium challenge. Collectively, studies establishing a mouse model for this coinfection suggest that multiple distinct malaria-induced immune defects contribute to increased susceptibility to S. Typhimurium.
Figures

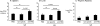
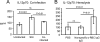
Similar articles
-
Malaria Parasite-Mediated Alteration of Macrophage Function and Increased Iron Availability Predispose to Disseminated Nontyphoidal Salmonella Infection.Infect Immun. 2018 Aug 22;86(9):e00301-18. doi: 10.1128/IAI.00301-18. Print 2018 Sep. Infect Immun. 2018. PMID: 29986892 Free PMC article.
-
Plasmodium-Salmonella Coinfection Induces Intense Inflammatory Response, Oxidative Stress, and Liver Damage: A Mice Model Study for Therapeutic Strategy.Shock. 2018 Dec;50(6):741-749. doi: 10.1097/SHK.0000000000001111. Shock. 2018. PMID: 29394238
-
Inflammation-associated alterations to the intestinal microbiota reduce colonization resistance against non-typhoidal Salmonella during concurrent malaria parasite infection.Sci Rep. 2015 Oct 5;5:14603. doi: 10.1038/srep14603. Sci Rep. 2015. PMID: 26434367 Free PMC article.
-
Malaria, anemia, and invasive bacterial disease: A neutrophil problem?J Leukoc Biol. 2019 Apr;105(4):645-655. doi: 10.1002/JLB.3RI1018-400R. Epub 2018 Dec 20. J Leukoc Biol. 2019. PMID: 30570786 Free PMC article. Review.
-
Immunological bases of increased susceptibility to invasive nontyphoidal Salmonella infection in children with malaria and anaemia.Microbes Infect. 2018 Oct-Nov;20(9-10):589-598. doi: 10.1016/j.micinf.2017.11.014. Epub 2017 Dec 15. Microbes Infect. 2018. PMID: 29248635 Free PMC article. Review.
Cited by
-
Host defense against malaria favors Salmonella.Nat Med. 2012 Jan 6;18(1):21-2. doi: 10.1038/nm.2636. Nat Med. 2012. PMID: 22227659 No abstract available.
-
Antibodies and Protection in Systemic Salmonella Infections: Do We Still Have More Questions than Answers?Infect Immun. 2020 Sep 18;88(10):e00219-20. doi: 10.1128/IAI.00219-20. Print 2020 Sep 18. Infect Immun. 2020. PMID: 32601109 Free PMC article. Review.
-
Transfusion of stored blood impairs host defenses against Gram-negative pathogens in mice.Transfusion. 2014 Nov;54(11):2842-51. doi: 10.1111/trf.12712. Epub 2014 May 19. Transfusion. 2014. PMID: 24840185 Free PMC article.
-
How Severe Anaemia Might Influence the Risk of Invasive Bacterial Infections in African Children.Int J Mol Sci. 2020 Sep 22;21(18):6976. doi: 10.3390/ijms21186976. Int J Mol Sci. 2020. PMID: 32972031 Free PMC article. Review.
-
Dissemination of non-typhoidal Salmonella during Plasmodium chabaudi infection affects anti-malarial immunity.Parasitol Res. 2019 Jul;118(7):2277-2285. doi: 10.1007/s00436-019-06349-z. Epub 2019 May 23. Parasitol Res. 2019. PMID: 31119381 Free PMC article.
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Benjamin, P. A., I. T. Ling, G. Clottey, L. M. Valero, S. A. Ogun, S. L. Fleck, D. Walliker, W. D. Morgan, B. Birdsall, J. Feeney, and A. A. Holder. 1999. Antigenic and sequence diversity at the C-terminus of the merozoite surface protein-1 from rodent malaria isolates, and the binding of protective monoclonal antibodies. Mol. Biochem. Parasitol. 104:147-156. - PubMed
-
- Brent, A. J., J. O. Oundo, I. Mwangi, L. Ochola, B. Lowe, and J. A. Berkley. 2006. Salmonella bacteremia in Kenyan children. Pediatr. Infect. Dis. J. 25:230-236. - PubMed
-
- Bronzan, R. N., T. E. Taylor, J. Mwenechanya, M. Tembo, K. Kayira, L. Bwanaisa, A. Njobvu, W. Kondowe, C. Chalira, A. L. Walsh, A. Phiri, L. K. Wilson, M. E. Molyneux, and S. M. Graham. 2007. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J. Infect. Dis. 195:895-904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

