Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation
- PMID: 20100866
- PMCID: PMC2832503
- DOI: 10.1128/MCB.01101-09
Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation
Erratum in
-
Correction for Fogal et al., "Mitochondrial p32 Protein Is a Critical Regulator of Tumor Metabolism via Maintenance of Oxidative Phosphorylation".Mol Cell Biol. 2017 Jun 29;37(14):e00137-17. doi: 10.1128/MCB.00137-17. Print 2017 Jul 15. Mol Cell Biol. 2017. PMID: 28663271 Free PMC article. No abstract available.
Abstract
p32/gC1qR/C1QBP/HABP1 is a mitochondrial/cell surface protein overexpressed in certain cancer cells. Here we show that knocking down p32 expression in human cancer cells strongly shifts their metabolism from oxidative phosphorylation (OXPHOS) to glycolysis. The p32 knockdown cells exhibited reduced synthesis of the mitochondrial-DNA-encoded OXPHOS polypeptides and were less tumorigenic in vivo. Expression of exogenous p32 in the knockdown cells restored the wild-type cellular phenotype and tumorigenicity. Increased glucose consumption and lactate production, known as the Warburg effect, are almost universal hallmarks of solid tumors and are thought to favor tumor growth. However, here we show that a protein regularly overexpressed in some cancers is capable of promoting OXPHOS. Our results indicate that high levels of glycolysis, in the absence of adequate OXPHOS, may not be as beneficial for tumor growth as generally thought and suggest that tumor cells use p32 to regulate the balance between OXPHOS and glycolysis.
Figures





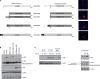




Comment in
-
p32 (C1QBP) and cancer cell metabolism: is the Warburg effect a lot of hot air?Mol Cell Biol. 2010 Mar;30(6):1300-2. doi: 10.1128/MCB.01661-09. Epub 2010 Jan 25. Mol Cell Biol. 2010. PMID: 20100868 Free PMC article. No abstract available.
References
-
- Alirol, E., and J. C. Martinou. 2006. Mitochondria and cancer: is there a morphological connection? Oncogene 25:4706-4716. - PubMed
-
- Biaglow, J. E., G. Cerniglia, S. Tuttle, V. Bakanauskas, C. Stevens, and G. McKenna. 1997. Effect of oncogene transformation of rat embryo cells on cellular oxygen consumption and glycolysis. Biochem. Biophys. Res. Commun. 235:739-742. - PubMed
-
- Caraux, G., and S. Pinloche. 2005. PermutMatrix: a graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 21:1280-1281. - PubMed
-
- Cavalli, L. R., M. Varella-Garcia, and B. C. Liang. 1997. Diminished tumorigenic phenotype after depletion of mitochondrial DNA. Cell Growth Differ. 8:1189-1198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
