A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation
- PMID: 20100867
- PMCID: PMC2838066
- DOI: 10.1128/MCB.01164-09
A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation
Abstract
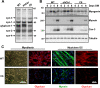
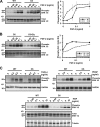
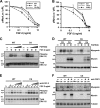
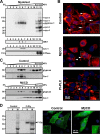
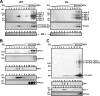
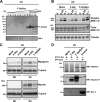
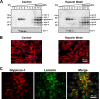
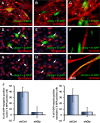
Heparan sulfate proteoglycans (HSPGs) are critical modulators of growth factor activities. Skeletal muscle differentiation is strongly inhibited by fibroblast growth factor 2 (FGF-2). We have shown that HSPGs present at the plasma membrane are expressed in myoblasts and are downregulated during muscle differentiation. An exception is glypican-1, which is present throughout the myogenic process. Myoblasts that do not express glypican-1 exhibit defective differentiation, with an increase in the receptor binding of FGF-2, concomitant with increased signaling. Glypican-1-deficient myoblasts show decreased expression of myogenin, the master gene that controls myogenesis, myosin, and the myoblast fusion index. Reversion of these defects was induced by expression of rat glypican-1. Glypican-1 is the only HSPG localized in lipid raft domains in myoblasts, resulting in the sequestration of FGF-2 away from FGF-2 receptors (FGFRs) located in nonraft domains. A chimeric glypican-1, containing syndecan-1 transmembrane and cytoplasmic domains, is located in nonraft domains interacting with FGFR-IV- and enhanced FGF-2-dependent signaling. Thus, glypican-1 acts as a positive regulator of muscle differentiation by sequestering FGF-2 in lipid rafts and preventing its binding and dependent signaling.
Figures


References
-
- Akiyama, S., T. Katagiri, M. Namiki, N. Yamaji, N. Yamamoto, K. Miyama, H. Shibuya, N. Ueno, J. M. Wozney, and T. Suda. 1997. Constitutively active BMP type I receptors transduce BMP-2 signals without the ligand in C2C12 myoblasts. Exp. Cell Res. 235:362-369. - PubMed
-
- Anastasi, S., S. Giordano, O. Sthandier, G. Gambarotta, R. Maione, P. Comoglio, and P. Amati. 1997. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J. Cell Biol. 137:1057-1068. - PMC - PubMed
-
- Ashikari, S., H. Habuchi, and K. Kimata. 1995. Characterization of heparan sulfate oligosaccharides that bind to hepatocyte growth factor. J. Biol. Chem. 270:29586-29593. - PubMed
-
- Bernfield, M., M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
