Production of a class II two-component lantibiotic of Streptococcus pneumoniae using the class I nisin synthetic machinery and leader sequence
- PMID: 20100873
- PMCID: PMC2849381
- DOI: 10.1128/AAC.00883-09
Production of a class II two-component lantibiotic of Streptococcus pneumoniae using the class I nisin synthetic machinery and leader sequence
Abstract
Recent studies showed that the nisin modification machinery can successfully dehydrate serines and threonines and introduce lanthionine rings in small peptides that are fused to the nisin leader sequence. This opens up exciting possibilities to produce and engineer larger antimicrobial peptides in vivo. Here we demonstrate the exploitation of the class I nisin production machinery to generate, modify, and secrete biologically active, previously not-yet-isolated and -characterized class II two-component lantibiotics that have no sequence homology to nisin. The nisin synthesis machinery, composed of the modification enzymes NisB and NisC and the transporter NisT, was used to modify and secrete a putative two-component lantibiotic of Streptococcus pneumoniae. This was achieved by genetically fusing the propeptide-encoding sequences of the spr1765 (pneA1) and spr1766 (pneA2) genes to the nisin leader-encoding sequence. The chimeric prepeptides were secreted out of Lactococcus lactis, purified by cation exchange fast protein liquid chromatography, and further characterized. Mass spectrometry analyses demonstrated the presence and partial localization of multiple dehydrated serines and/or threonines and (methyl)lanthionines in both peptides. Moreover, after cleavage of the leader peptide from the prepeptides, both modified propeptides displayed antimicrobial activity against Micrococcus flavus. These results demonstrate that the nisin synthetase machinery can be successfully used to modify and produce otherwise difficult to obtain antimicrobially active lantibiotics.
Figures


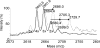
References
-
- Chakicherla, A., and J. N. Hansen.1995. Role of the leader and structural regions of prelantibiotic peptides as assessed by expressing nisin-subtilin chimeras in Bacillus subtilis 168, and characterization of their physical, chemical, and antimicrobial properties. J. Biol. Chem. 270:23533-23539. - PubMed
-
- Chatterjee, C., G. C. Patton, L. Cooper, M. Paul, and W. A. van der Donk.2006. Engineering dehydro amino acids and thioethers into peptides using lacticin 481 synthetase. Chem. Biol. 13:1109-1117. - PubMed
-
- Chatterjee, C., M. Paul, L. Xie, and W. A. van der Donk.2005. Biosynthesis and mode of action of lantibiotics. Chem. Rev. 105:633-684. - PubMed
-
- Reference deleted.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

