Immunomodulation of voltage-dependent K+ channels in macrophages: molecular and biophysical consequences
- PMID: 20100893
- PMCID: PMC2812499
- DOI: 10.1085/jgp.200910334
Immunomodulation of voltage-dependent K+ channels in macrophages: molecular and biophysical consequences
Abstract
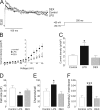
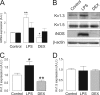
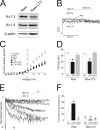
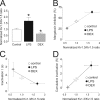
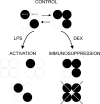
Voltage-dependent potassium (K(v)) channels play a pivotal role in the modulation of macrophage physiology. Macrophages are professional antigen-presenting cells and produce inflammatory and immunoactive substances that modulate the immune response. Blockage of K(v) channels by specific antagonists decreases macrophage cytokine production and inhibits proliferation. Numerous pharmacological agents exert their effects on specific target cells by modifying the activity of their plasma membrane ion channels. Investigation of the mechanisms involved in the regulation of potassium ion conduction is, therefore, essential to the understanding of potassium channel functions in the immune response to infection and inflammation. Here, we demonstrate that the biophysical properties of voltage-dependent K(+) currents are modified upon activation or immunosuppression in macrophages. This regulation is in accordance with changes in the molecular characteristics of the heterotetrameric K(v)1.3/K(v)1.5 channels, which generate the main K(v) in macrophages. An increase in K(+) current amplitude in lipopolysaccharide-activated macrophages is characterized by a faster C-type inactivation, a greater percentage of cumulative inactivation, and a more effective margatoxin (MgTx) inhibition than control cells. These biophysical parameters are related to an increase in K(v)1.3 subunits in the K(v)1.3/K(v)1.5 hybrid channel. In contrast, dexamethasone decreased the C-type inactivation, the cumulative inactivation, and the sensitivity to MgTx concomitantly with a decrease in K(v)1.3 expression. Neither of these treatments apparently altered the expression of K(v)1.5. Our results demonstrate that the immunomodulation of macrophages triggers molecular and biophysical consequences in K(v)1.3/K(v)1.5 hybrid channels by altering the subunit stoichiometry.
Figures

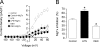
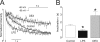

Similar articles
-
Kv1.3/Kv1.5 heteromeric channels compromise pharmacological responses in macrophages.Biochem Biophys Res Commun. 2007 Jan 26;352(4):913-8. doi: 10.1016/j.bbrc.2006.11.120. Epub 2006 Dec 4. Biochem Biophys Res Commun. 2007. PMID: 17157812
-
Association of Kv1.5 and Kv1.3 contributes to the major voltage-dependent K+ channel in macrophages.J Biol Chem. 2006 Dec 8;281(49):37675-85. doi: 10.1074/jbc.M605617200. Epub 2006 Oct 11. J Biol Chem. 2006. PMID: 17038323
-
Modulation of voltage-dependent and inward rectifier potassium channels by 15-epi-lipoxin-A4 in activated murine macrophages: implications in innate immunity.J Immunol. 2013 Dec 15;191(12):6136-46. doi: 10.4049/jimmunol.1300235. Epub 2013 Nov 18. J Immunol. 2013. PMID: 24249731
-
The secret life of ion channels: Kv1.3 potassium channels and proliferation.Am J Physiol Cell Physiol. 2018 Jan 1;314(1):C27-C42. doi: 10.1152/ajpcell.00136.2017. Epub 2017 Sep 20. Am J Physiol Cell Physiol. 2018. PMID: 28931540 Review.
-
Potassium channel-blockers as therapeutic agents to interfere with bone resorption of periodontal disease.J Dent Res. 2005 Jun;84(6):488-99. doi: 10.1177/154405910508400603. J Dent Res. 2005. PMID: 15914584 Review.
Cited by
-
Novel insights into the electrophysiology of murine cardiac macrophages: relevance of voltage-gated potassium channels.Cardiovasc Res. 2022 Feb 21;118(3):798-813. doi: 10.1093/cvr/cvab126. Cardiovasc Res. 2022. PMID: 33823533 Free PMC article.
-
The Potassium Channel Kv1.5 Expression Alters During Experimental Autoimmune Encephalomyelitis.Neurochem Res. 2019 Dec;44(12):2733-2745. doi: 10.1007/s11064-019-02892-4. Epub 2019 Oct 17. Neurochem Res. 2019. PMID: 31624998
-
Effect of Voltage-Gated K+ Channel Inhibition by 4-aminopyridine in Spinal Cord Injury Recovery in Zebrafish.bioRxiv [Preprint]. 2024 Jul 18:2024.07.15.603582. doi: 10.1101/2024.07.15.603582. bioRxiv. 2024. PMID: 39071260 Free PMC article. Preprint.
-
Kv1.5 in the immune system: the good, the bad, or the ugly?Front Physiol. 2010 Nov 16;1:152. doi: 10.3389/fphys.2010.00152. eCollection 2010. Front Physiol. 2010. PMID: 21423392 Free PMC article.
-
Bioelectric modulation of macrophage polarization.Sci Rep. 2016 Feb 12;6:21044. doi: 10.1038/srep21044. Sci Rep. 2016. PMID: 26869018 Free PMC article.

