Intrinsic versus extrinsic voltage sensitivity of blocker interaction with an ion channel pore
- PMID: 20100894
- PMCID: PMC2812505
- DOI: 10.1085/jgp.200910324
Intrinsic versus extrinsic voltage sensitivity of blocker interaction with an ion channel pore
Abstract
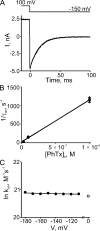
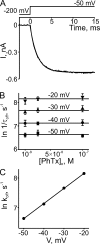
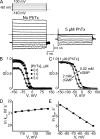
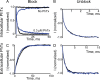
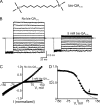
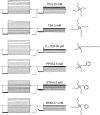
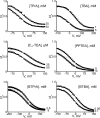
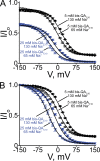
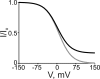
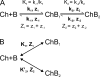
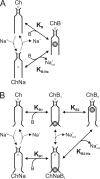
Many physiological and synthetic agents act by occluding the ion conduction pore of ion channels. A hallmark of charged blockers is that their apparent affinity for the pore usually varies with membrane voltage. Two models have been proposed to explain this voltage sensitivity. One model assumes that the charged blocker itself directly senses the transmembrane electric field, i.e., that blocker binding is intrinsically voltage dependent. In the alternative model, the blocker does not directly interact with the electric field; instead, blocker binding acquires voltage dependence solely through the concurrent movement of permeant ions across the field. This latter model may better explain voltage dependence of channel block by large organic compounds that are too bulky to fit into the narrow (usually ion-selective) part of the pore where the electric field is steep. To date, no systematic investigation has been performed to distinguish between these voltage-dependent mechanisms of channel block. The most fundamental characteristic of the extrinsic mechanism, i.e., that block can be rendered voltage independent, remains to be established and formally analyzed for the case of organic blockers. Here, we observe that the voltage dependence of block of a cyclic nucleotide-gated channel by a series of intracellular quaternary ammonium blockers, which are too bulky to traverse the narrow ion selectivity filter, gradually vanishes with extreme depolarization, a predicted feature of the extrinsic voltage dependence model. In contrast, the voltage dependence of block by an amine blocker, which has a smaller "diameter" and can therefore penetrate into the selectivity filter, follows a Boltzmann function, a predicted feature of the intrinsic voltage dependence model. Additionally, a blocker generates (at least) two blocked states, which, if related serially, may preclude meaningful application of a commonly used approach for investigating channel gating, namely, inferring the properties of the activation gate from the kinetics of channel block.
Figures




Similar articles
-
Mechanism of the voltage sensitivity of IRK1 inward-rectifier K+ channel block by the polyamine spermine.J Gen Physiol. 2005 Apr;125(4):413-26. doi: 10.1085/jgp.200409242. Epub 2005 Mar 14. J Gen Physiol. 2005. PMID: 15795311 Free PMC article.
-
State-independent block of BK channels by an intracellular quaternary ammonium.J Gen Physiol. 2006 Sep;128(3):347-64. doi: 10.1085/jgp.200609579. J Gen Physiol. 2006. PMID: 16940557 Free PMC article.
-
New insights on the voltage dependence of the KCa3.1 channel block by internal TBA.J Gen Physiol. 2004 Oct;124(4):333-48. doi: 10.1085/jgp.200409145. J Gen Physiol. 2004. PMID: 15452196 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Pharmacology of voltage-gated proton channels.Curr Pharm Des. 2007;13(23):2400-20. doi: 10.2174/138161207781368675. Curr Pharm Des. 2007. PMID: 17692009 Review.
Cited by
-
K⁺ conduction and Mg²⁺ blockade in a shaker Kv-channel single point mutant with an unusually high conductance.Biophys J. 2012 Sep 19;103(6):1198-207. doi: 10.1016/j.bpj.2012.08.015. Biophys J. 2012. PMID: 22995492 Free PMC article.
-
Ouabain binding site in a functioning Na+/K+ ATPase.J Biol Chem. 2011 Nov 4;286(44):38177-38183. doi: 10.1074/jbc.M111.267682. Epub 2011 Sep 12. J Biol Chem. 2011. PMID: 21911500 Free PMC article.
-
Voltage profile along the permeation pathway of an open channel.Biophys J. 2010 Nov 3;99(9):2863-9. doi: 10.1016/j.bpj.2010.08.053. Biophys J. 2010. PMID: 21044583 Free PMC article.
-
The voltage-dependent gate in MthK potassium channels is located at the selectivity filter.Nat Struct Mol Biol. 2013 Feb;20(2):159-66. doi: 10.1038/nsmb.2473. Epub 2012 Dec 23. Nat Struct Mol Biol. 2013. PMID: 23262489 Free PMC article.
-
Mechanisms for Kir channel inhibition by quinacrine: acute pore block of Kir2.x channels and interference in PIP2 interaction with Kir2.x and Kir6.2 channels.Pflugers Arch. 2011 Oct;462(4):505-17. doi: 10.1007/s00424-011-0995-5. Epub 2011 Jul 22. Pflugers Arch. 2011. PMID: 21779761
References
-
- Becchetti A., Roncaglia P. 2000. Cyclic nucleotide-gated channels: intra- and extracellular accessibility to Cd2+ of substituted cysteine residues within the P-loop. PflÜgers Arch. 440:556–565 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

