Enhanced sensitivity of pancreatic cancer cells to concurrent inhibition of aberrant signal transducer and activator of transcription 3 and epidermal growth factor receptor or Src
- PMID: 20100905
- PMCID: PMC2872953
- DOI: 10.1124/jpet.109.162669
Enhanced sensitivity of pancreatic cancer cells to concurrent inhibition of aberrant signal transducer and activator of transcription 3 and epidermal growth factor receptor or Src
Abstract
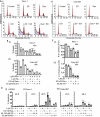
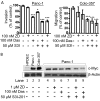
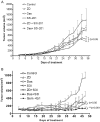
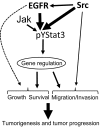
Many molecular aberrations occur in pancreatic cancer. Although aberrant epidermal growth factor receptor (EGFR), Src, and signal transducer and activator of transcription 3 (Stat3) are implicated in pancreatic cancer, therapies that target only one of these entities are undermined by signaling cross-talk. In the human pancreatic cancer lines, Panc-1 and Colo-357, pY845EGFR, pY1068EGFR, pY1086EGFR, and pY1173EGFR levels and pY416c-Src are concurrently elevated with aberrantly active Stat3 in a complex signaling cross-talk. Thus, understanding the signaling integration would facilitate the design of effective multiple-targeted therapeutic modalities. In Panc-1 and Colo-357 lines, pY845EGFR, pY1068EGFR, and pY1086EGFR levels are responsive to c-Src inhibition in contrast to pY1173EGFR, which is EGFR kinase-dependent. Constitutively active Stat3 is sensitive to both EGFR and Src inhibition, but the early suppression of aberrantly active Stat3 in response to the inhibition of EGFR and Src is countered by a Janus kinase (Jaks)-dependent reactivation, suggesting that Jaks activity is a compensatory mechanism for Stat3 induction. The inhibition of EGFR, Src, or Stat3 alone induced weak biological responses. By contrast, the concurrent inhibition of Stat3 and EGFR or Src induced greater viability loss and apoptosis and decreased the migration/invasion of pancreatic cancer cells in vitro. Significantly, the concurrent inhibition, compared with monotargeting modality, induced stronger human pancreatic tumor growth inhibition in xenografts. We infer that the tumor growth inhibition in vivo is caused by the simultaneous suppression of the abnormal functions of Stat3 and EGFR or Src. These studies strongly suggest that the concurrent targeting of Stat3 and EGFR or Src could be a beneficial therapeutic approach for pancreatic cancer.
Figures



Similar articles
-
Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth.Clin Cancer Res. 2011 Feb 1;17(3):483-93. doi: 10.1158/1078-0432.CCR-10-1670. Epub 2011 Jan 25. Clin Cancer Res. 2011. PMID: 21266529 Free PMC article.
-
Dual inhibition of Janus and Src family kinases by novel indirubin derivative blocks constitutively-activated Stat3 signaling associated with apoptosis of human pancreatic cancer cells.Mol Oncol. 2013 Jun;7(3):369-78. doi: 10.1016/j.molonc.2012.10.013. Epub 2012 Nov 16. Mol Oncol. 2013. PMID: 23206899 Free PMC article.
-
Pyk2 amplifies epidermal growth factor and c-Src-induced Stat3 activation.J Biol Chem. 2004 Apr 23;279(17):17224-31. doi: 10.1074/jbc.M311875200. Epub 2004 Feb 12. J Biol Chem. 2004. PMID: 14963038
-
Crosstalk of Sp1 and Stat3 signaling in pancreatic cancer pathogenesis.Cytokine Growth Factor Rev. 2012 Feb-Apr;23(1-2):25-35. doi: 10.1016/j.cytogfr.2012.01.003. Epub 2012 Feb 16. Cytokine Growth Factor Rev. 2012. PMID: 22342309 Free PMC article. Review.
-
Pathways of metastasis suppression in bladder cancer.Cancer Metastasis Rev. 2009 Dec;28(3-4):327-33. doi: 10.1007/s10555-009-9197-4. Cancer Metastasis Rev. 2009. PMID: 20013033 Review.
Cited by
-
Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1α in EGFR-mutated lung cancer in vitro and in vivo.Neoplasia. 2015 Feb;17(2):190-200. doi: 10.1016/j.neo.2014.12.008. Neoplasia. 2015. PMID: 25748238 Free PMC article.
-
Docoxahexaenoic Acid Induces Apoptosis of Pancreatic Cancer Cells by Suppressing Activation of STAT3 and NF-κB.Nutrients. 2018 Nov 2;10(11):1621. doi: 10.3390/nu10111621. Nutrients. 2018. PMID: 30400136 Free PMC article.
-
A cell-permeable Stat3 SH2 domain mimetic inhibits Stat3 activation and induces antitumor cell effects in vitro.J Biol Chem. 2010 Nov 12;285(46):35855-65. doi: 10.1074/jbc.M110.154088. Epub 2010 Aug 31. J Biol Chem. 2010. PMID: 20807764 Free PMC article.
-
DEspR roles in tumor vasculo-angiogenesis, invasiveness, CSC-survival and anoikis resistance: a 'common receptor coordinator' paradigm.PLoS One. 2014 Jan 21;9(1):e85821. doi: 10.1371/journal.pone.0085821. eCollection 2014. PLoS One. 2014. PMID: 24465725 Free PMC article.
-
Functional roles of SRC signaling in pancreatic cancer: Recent insights provide novel therapeutic opportunities.Oncogene. 2023 Jun;42(22):1786-1801. doi: 10.1038/s41388-023-02701-x. Epub 2023 Apr 29. Oncogene. 2023. PMID: 37120696 Free PMC article. Review.
References
-
- Burris H, 3rd, Rocha-Lima C. (2008) New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist 13:289–298 - PubMed
-
- Darnell JE. (2005) Validating Stat3 in cancer therapy. Nat Med 11:595–596 - PubMed
-
- DeArmond D, Brattain MG, Jessup JM, Kreisberg J, Malik S, Zhao S, Freeman JW. (2003) Autocrine-mediated ErbB-2 kinase activation of STAT3 is required for growth factor independence of pancreatic cancer cell lines. Oncogene 22:7781–7795 - PubMed
-
- Dong M, Nio Y, Guo KJ, Tamura K, Tian YL, Dong YT. (1998) Epidermal growth factor and its receptor as prognostic indicators in Chinese patients with pancreatic cancer. Anticancer Res 18:4613–4619 - PubMed
-
- Downward J, Parker P, Waterfield MD. (1984) Autophosphorylation sites on the epidermal growth factor receptor. Nature 311:483–485 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

