Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer
- PMID: 20100959
- PMCID: PMC2834462
- DOI: 10.1200/JCO.2009.25.0597
Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer
Abstract
Purpose: Therapeutic prostate-specific antigen (PSA) -targeted poxviral vaccines for prostate cancer have been well tolerated. PROSTVAC-VF treatment was evaluated for safety and for prolongation of progression-free survival (PFS) and overall survival (OS) in a randomized, controlled, and blinded phase II study.
Patients and methods: In total, 125 patients were randomly assigned in a multicenter trial of vaccination series. Eligible patients had minimally symptomatic castration-resistant metastatic prostate cancer (mCRPC). PROSTVAC-VF comprises two recombinant viral vectors, each encoding transgenes for PSA, and three immune costimulatory molecules (B7.1, ICAM-1, and LFA-3). Vaccinia-based vector was used for priming followed by six planned fowlpox-based vector boosts. Patients were allocated (2:1) to PROSTVAC-VF plus granulocyte-macrophage colony-stimulating factor or to control empty vectors plus saline injections.
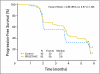
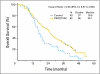
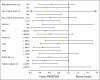
Results: Eighty-two patients received PROSTVAC-VF and 40 received control vectors. Patient characteristics were similar in both groups. The primary end point was PFS, which was similar in the two groups (P = .6). However, at 3 years post study, PROSTVAC-VF patients had a better OS with 25 (30%) of 82 alive versus 7 (17%) of 40 controls, longer median survival by 8.5 months (25.1 v 16.6 months for controls), an estimated hazard ratio of 0.56 (95% CI, 0.37 to 0.85), and stratified log-rank P = .0061.
Conclusion: PROSTVAC-VF immunotherapy was well tolerated and associated with a 44% reduction in the death rate and an 8.5-month improvement in median OS in men with mCRPC. These provocative data provide preliminary evidence of clinically meaningful benefit but need to be confirmed in a larger phase III study.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Developing immunotherapy as legitimate therapy for patients with prostate cancer.J Clin Oncol. 2010 Mar 1;28(7):1085-7. doi: 10.1200/JCO.2009.26.3483. Epub 2010 Jan 25. J Clin Oncol. 2010. PMID: 20100956 No abstract available.
-
Poxviral-based prostate-specific antigen vaccine in prostate cancer.J Clin Oncol. 2010 Aug 20;28(24):e416; author reply e417. doi: 10.1200/JCO.2010.29.1070. Epub 2010 Jun 21. J Clin Oncol. 2010. PMID: 20566998 No abstract available.
-
Words of wisdom. Re: Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. Kantoff PW, Schuetz TJ, Blumenstein BA, et al.Eur Urol. 2010 Sep;58(3):466. doi: 10.1016/j.eururo.2010.06.020. Eur Urol. 2010. PMID: 20845530 No abstract available.
-
Words of wisdom. Re: overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer.Eur Urol. 2010 Oct;58(4):632-3. doi: 10.1016/j.eururo.2010.07.016. Eur Urol. 2010. PMID: 20848748 No abstract available.
References
-
- Sanda MG, Smith DC, Linda GC, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. - PubMed
-
- Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. - PubMed
-
- Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–117. - PubMed
-
- Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): A trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. - PubMed
-
- Hodge JW, Sabzevari H, Yafal AG, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

