Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer
- PMID: 20100965
- PMCID: PMC2834466
- DOI: 10.1200/JCO.2009.22.4725
Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer
Abstract
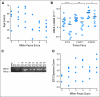
PURPOSE Cisplatin is a chemotherapeutic agent not used routinely for breast cancer treatment. As a DNA cross-linking agent, cisplatin may be effective treatment for hereditary BRCA1-mutated breast cancers. Because sporadic triple-negative breast cancer (TNBC) and BRCA1-associated breast cancer share features suggesting common pathogenesis, we conducted a neoadjuvant trial of cisplatin in TNBC and explored specific biomarkers to identify predictors of response. PATIENTS AND METHODS Twenty-eight women with stage II or III breast cancers lacking estrogen and progesterone receptors and HER2/Neu (TNBC) were enrolled and treated with four cycles of cisplatin at 75 mg/m(2) every 21 days. After definitive surgery, patients received standard adjuvant chemotherapy and radiation therapy per their treating physicians. Clinical and pathologic treatment response were assessed, and pretreatment tumor samples were evaluated for selected biomarkers. Results Six (22%) of 28 patients achieved pathologic complete responses, including both patients with BRCA1 germline mutations;18 (64%) patients had a clinical complete or partial response. Fourteen (50%) patients showed good pathologic responses (Miller-Payne score of 3, 4, or 5), 10 had minor responses (Miller-Payne score of 1 or 2), and four (14%) progressed. All TNBCs clustered with reference basal-like tumors by hierarchical clustering. Factors associated with good cisplatin response include young age (P = .001), low BRCA1 mRNA expression (P = .03), BRCA1 promoter methylation (P = .04), p53 nonsense or frameshift mutations (P = .01), and a gene expression signature of E2F3 activation (P = .03). CONCLUSION Single-agent cisplatin induced response in a subset of patients with TNBC. Decreased BRCA1 expression may identify subsets of TNBCs that are cisplatin sensitive. Other biomarkers show promise in predicting cisplatin response.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–1281. - PubMed
-
- Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. - PubMed
-
- Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–5685. - PubMed
-
- Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95:1482–1485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

