Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease
- PMID: 20101004
- PMCID: PMC2872973
- DOI: 10.1124/mol.109.061754
Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease
Abstract
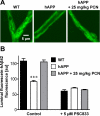
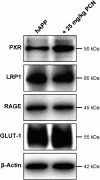
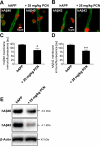
Reduced clearance of amyloid-beta (Abeta) from brain partly underlies increased Abeta brain accumulation in Alzheimer's disease (AD). The mechanistic basis for this pathology is unknown, but recent evidence suggests a neurovascular component in AD etiology. We show here that the ATP-driven pump, P-glycoprotein, specifically mediates efflux transport of Abeta from mouse brain capillaries into the vascular space, thus identifying a critical component of the Abeta brain efflux mechanism. We demonstrate in a transgenic mouse model of AD [human amyloid precursor protein (hAPP)-overexpressing mice; Tg2576 strain] that brain capillary P-glycoprotein expression and transport activity are substantially reduced compared with wild-type control mice, suggesting a mechanism by which Abeta accumulates in the brain in AD. It is noteworthy that dosing 12-week-old, asymptomatic hAPP mice over 7 days with pregnenolone-16alpha-carbonitrile to activate the nuclear receptor pregnane X receptor restores P-glycoprotein expression and transport activity in brain capillaries and significantly reduces brain Abeta levels compared with untreated control mice. Thus, targeting intracellular signals that up-regulate blood-brain barrier P-glycoprotein in the early stages of AD has the potential to increase Abeta clearance from the brain and reduce Abeta brain accumulation. This mechanism suggests a new therapeutic strategy in AD.
Figures




References
-
- Bauer B, Hartz AM, Fricker G, Miller DS. (2004) Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 66:413–419 - PubMed
-
- Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS. (2008) Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab 28:1222–1234 - PubMed
-
- Bauer B, Yang X, Hartz AM, Olson ER, Zhao R, Kalvass JC, Pollack GM, Miller DS. (2006) In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol Pharmacol 70:1212–1219 - PubMed
-
- Blennow K, de Leon MJ, Zetterberg H. (2006) Alzheimer's disease. Lancet 368:387–403 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

