TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice
- PMID: 20101093
- PMCID: PMC2810071
- DOI: 10.1172/JCI38136
TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice
Abstract
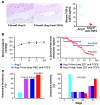
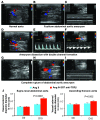
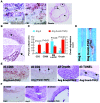
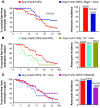
Complicated abdominal aortic aneurysm (AAA) is a major cause of mortality in elderly men. Ang II-dependent TGF-beta activity promotes aortic aneurysm progression in experimental Marfan syndrome. However, the role of TGF-beta in experimental models of AAA has not been comprehensively assessed. Here, we show that systemic neutralization of TGF-beta activity breaks the resistance of normocholesterolemic C57BL/6 mice to Ang II-induced AAA formation and markedly increases their susceptibility to the disease. These aneurysms displayed a large spectrum of complications on echography, including fissuration, double channel formation, and rupture, leading to death from aneurysm complications. The disease was refractory to inhibition of IFN-gamma, IL-4, IL-6, or TNF-alpha signaling. Genetic deletion of T and B cells or inhibition of the CX3CR1 pathway resulted in partial protection. Interestingly, neutralization of TGF-beta activity enhanced monocyte invasiveness, and monocyte depletion markedly inhibited aneurysm progression and complications. Finally, TGF-beta neutralization increased MMP-12 activity, and MMP-12 deficiency prevented aneurysm rupture. These results clearly identify a critical role for TGF-beta in the taming of the innate immune response and the preservation of vessel integrity in C57BL/6 mice, which contrasts with its reported pathogenic role in Marfan syndrome.
Figures


Comment in
-
TGF-beta in the pathogenesis and prevention of disease: a matter of aneurysmic proportions.J Clin Invest. 2010 Feb;120(2):403-7. doi: 10.1172/JCI42014. Epub 2010 Jan 25. J Clin Invest. 2010. PMID: 20101091 Free PMC article.
References
-
- Best VA, Price JF, Fowkes FG. Persistent increase in the incidence of abdominal aortic aneurysm in Scotland, 1981-2000. Br J Surg. 2003;90(12):1510–1515. - PubMed
-
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142(3):203–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

