Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice
- PMID: 20101095
- PMCID: PMC2811160
- DOI: 10.1172/JCI39293
Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice
Abstract
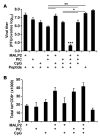
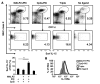
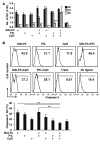
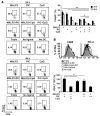
TLR ligands are promising candidates for the development of novel vaccine adjuvants that can elicit protective immunity against emerging infectious diseases. Adjuvants have been used most frequently to increase the quantity of an immune response. However, the quality of a T cell response can be more important than its quantity. Stimulating certain pairs of TLRs induces a synergistic response in terms of activating dendritic cells and eliciting/enhancing T cell responses through clonal expansion, which increases the number of responding T cells. Here, we have found that utilizing ligands for 3 TLRs (TLR2/6, TLR3, and TLR9) greatly increased the protective efficacy of vaccination with an HIV envelope peptide in mice when compared with using ligands for only any 2 of these TLRs; surprisingly, increased protection was induced without a marked increase in the number of peptide-specific T cells. Rather, the combination of these 3 TLR ligands augmented the quality of the T cell responses primarily by amplifying their functional avidity for the antigen, which was necessary for clearance of virus. The triple combination increased production of DC IL-15 along with its receptor, IL-15Ralpha, which contributed to high avidity, and decreased expression of programmed death-ligand 1 and induction of Tregs. Therefore, selective TLR ligand combinations can increase protective efficacy by increasing the quality rather than the quantity of T cell responses.
Figures


References
-
- Granucci F, Ricciardi-Castagnoli P. Interactions of bacterial pathogens with dendritic cells during invasion of mucosal surfaces. Curr Opin Microbiol. 2003;6(1):72–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

