TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma
- PMID: 20101235
- PMCID: PMC3138334
- DOI: 10.1038/onc.2009.486
TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma
Abstract
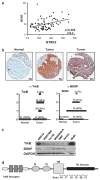
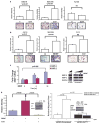
Head and neck squamous cell carcinoma (HNSCC) remains a significant public health problem, accounting for over 5% of all cancer-related deaths, and these deaths primarily result from metastatic disease. The molecular processes involved in HNSCC pathogenesis and progression are poorly understood, and here we present experimental evidence for a direct role of the cell surface receptor tyrosine kinase, TrkB, in HNSCC tumor progression. Using immunohistochemical analysis and transcriptional profiling of archival HNSCC tumor specimens, we found that TrkB and its secreted ligand, brain-derived neurotrophic factor (BDNF), are expresses in greater than 50% of human HNSCC tumors, but not in normal upper aerodigestive tract (UADT) epithelia. Studies with HNSCC cell lines reveal that in vitro stimulation with BDNF, the ligand for TrkB, upregulates the migration and invasion of HNSCC cells, and both transient and stable suppressions of TrkB result in significant abrogation of constitutive and ligand-mediated migration and invasion. Furthermore, enforced overexpression of TrkB results in altered expression of molecular mediators of epithelial-to-mesenchymal transition (EMT), including downregulation of E-cadherin and upregulation of Twist. Using an in vivo mouse model of HNSCC, we were able to show that downregulation of TrkB suppresses tumor growth. These results directly implicate TrkB in EMT and the invasive behavior of HNSCC, and correlate with the in vivo overexpression of TrkB in human HNSCC. Taken together, these data suggest that the TrkB receptor may be a critical component in the multi-step tumor progression of HNSCC, and may be an attractive target for much needed new therapies for this disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Brain-Derived Neurotrophin and TrkB in Head and Neck Squamous Cell Carcinoma.Int J Mol Sci. 2019 Jan 11;20(2):272. doi: 10.3390/ijms20020272. Int J Mol Sci. 2019. PMID: 30641914 Free PMC article.
-
Implications of tropomyosin-related kinase B (TrkB) in head and neck cancer.Anticancer Res. 2007 Sep-Oct;27(5A):3121-6. Anticancer Res. 2007. PMID: 17970052
-
Upregulation of TrkB promotes epithelial-mesenchymal transition and anoikis resistance in endometrial carcinoma.PLoS One. 2013 Jul 30;8(7):e70616. doi: 10.1371/journal.pone.0070616. Print 2013. PLoS One. 2013. PMID: 23936232 Free PMC article.
-
Uncovering the role of brain-derived neurotrophic factor/tyrosine kinase receptor B signaling in head and neck malignancies.J Oral Pathol Med. 2018 Mar;47(3):221-227. doi: 10.1111/jop.12611. Epub 2017 Jul 22. J Oral Pathol Med. 2018. PMID: 28650560 Review.
-
Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma.J Dent Res. 2013 Feb;92(2):114-21. doi: 10.1177/0022034512467352. Epub 2012 Nov 5. J Dent Res. 2013. PMID: 23128109 Free PMC article. Review.
Cited by
-
NeuroD1 regulates survival and migration of neuroendocrine lung carcinomas via signaling molecules TrkB and NCAM.Proc Natl Acad Sci U S A. 2013 Apr 16;110(16):6524-9. doi: 10.1073/pnas.1303932110. Epub 2013 Apr 3. Proc Natl Acad Sci U S A. 2013. PMID: 23553831 Free PMC article.
-
Stromal contributions to the carcinogenic process.Mol Carcinog. 2017 Apr;56(4):1199-1213. doi: 10.1002/mc.22583. Epub 2016 Nov 5. Mol Carcinog. 2017. PMID: 27787930 Free PMC article. Review.
-
Relevance of Neurotrophin Receptors CD271 and TrkC for Prognosis, Migration, and Proliferation in Head and Neck Squamous Cell Carcinoma.Cells. 2019 Sep 28;8(10):1167. doi: 10.3390/cells8101167. Cells. 2019. PMID: 31569361 Free PMC article.
-
Investigating Trk Protein Expression between Oropharyngeal and Non-oropharyngeal Squamous Cell Carcinoma: Clinical Implications and Possible Roles of Human Papillomavirus Infection.Cancer Res Treat. 2019 Jul;51(3):1052-1063. doi: 10.4143/crt.2018.411. Epub 2018 Oct 24. Cancer Res Treat. 2019. PMID: 30360033 Free PMC article.
-
Perineural Invasion in Head and Neck Cancer.J Dent Res. 2018 Jul;97(7):742-750. doi: 10.1177/0022034518756297. Epub 2018 Feb 14. J Dent Res. 2018. PMID: 29443582 Free PMC article. Review.
References
-
- Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001;105:425–431. - PubMed
-
- Armistead P, Salganick J, Roh J, Steinert D, Patel S, Munsell M, et al. Expression of receptor tyrosine kinases and apoptotic molecules in rhabdomyosarcoma. Cancer. 2007;110:2293–2303. - PubMed
-
- Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, Saha S, et al. Mutational analysis of the tyrosine kinome in colorectal cancers. Science. 2003;300:949. - PubMed
-
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. - PubMed
-
- Chao MV, Lee FS. Neurotrophin survival signaling mechanisms. J Alzheimers Dis. 2004;6:S7–S11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

