Efficacy and tolerability of cefditoren pivoxil in uncomplicated skin and skin structure infections in Indian patients
- PMID: 20101337
- PMCID: PMC2807712
- DOI: 10.4103/0019-5154.57612
Efficacy and tolerability of cefditoren pivoxil in uncomplicated skin and skin structure infections in Indian patients
Abstract
Background: Uncomplicated skin and skin structure infections (uSSSI) are commonly encountered community-acquired infections and are typically confined to the superficial layers of the skin. Hence, they seldom lead to the destruction of skin structures.
Aims: To evaluate the efficacy and tolerability of cefditoren pivoxil in uSSSI in Indian patients.
Methods: One hundred and seventy-eight patients diagnosed with uncomplicated SSSI were enrolled in this randomized, comparative, multicentric study. Patients received either cefditoren pivoxil or cefdinir for ten days. Efficacy was assessed both clinically and microbiologically. Safety evaluation consisted of reporting of type, frequency, severity, and causal relationship of adverse events.
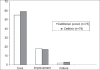
Results: One hundred and fifty-one patients completed the study. Clinical and bacteriological efficacy of cefditoren pivoxil was comparable to that of cefdinir in the treatment of uSSSI. One hundred and five patients were eligible for per protocol (PP) analysis of bacteriological outcome and clinical efficacy. Clinical cure or improvement was achieved in 98.00% patients treated with cefditoren pivoxil and 98.18% patients treated with cefdinir. In the modified Intent to Treat (mITT) patient population, clinical cure or improvement was recorded in 97.33% patients treated with cefditoren pivoxil and 96.20% patients treated with cefdinir. Microbiological eradication (or presumed eradication) was recorded in 88.00% patients treated with cefditoren pivoxil and 94.55% patients treated with cefdinir. The above differences in the outcome rates between the two drugs were not statistically significant. Six adverse events (AEs) (two in cefditoren group and four in cefdinir group) were reported in this study.
Conclusion: Cefditoren pivoxil 200 mg b.i.d. was effective and well tolerated in the treatment of uSSSI.
Keywords: Cefditoren pivoxil; uSSSI; uncomplicated skin and skin structure infection.
Conflict of interest statement
Figures


References
-
- Bucko AD, Hunt BJ, Kidd SL, Hom R. Randomized, double-blind, multicenter comparison of oral cefditoren 200 or 400 mg BID with either cefuroxime 250 mg BID or cefadroxil 500 mg BID for the treatment of uncomplicated skin and skin-structure infections. Clin Ther. 2002;24:1134–47. - PubMed
-
- Darkes MJM, Plosker GL. Cefditoren pivoxil. Drugs. 2002;62:319–36. - PubMed
-
- Wellington K, Curran MP. Cefditoren pivoxil: A review of its use in the treatment of bacterial infections. Drugs. 2004;64:2597–618. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
