Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP
- PMID: 20101601
- PMCID: PMC2891364
- DOI: 10.1002/hipo.20768
Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP
Abstract
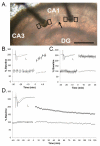
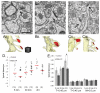
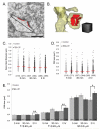
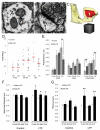
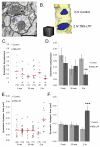
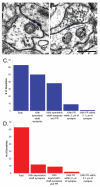
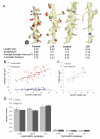
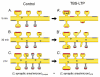
Enlargement of dendritic spines and synapses correlates with enhanced synaptic strength during long-term potentiation (LTP), especially in immature hippocampal neurons. Less clear is the nature of this structural synaptic plasticity on mature hippocampal neurons, and nothing is known about the structural plasticity of inhibitory synapses during LTP. Here the timing and extent of structural synaptic plasticity and changes in local protein synthesis evidenced by polyribosomes were systematically evaluated at both excitatory and inhibitory synapses on CA1 dendrites from mature rats following induction of LTP with theta-burst stimulation (TBS). Recent work suggests dendritic segments can act as functional units of plasticity. To test whether structural synaptic plasticity is similarly coordinated, we reconstructed from serial section transmission electron microscopy all of the spines and synapses along representative dendritic segments receiving control stimulation or TBS-LTP. At 5 min after TBS, polyribosomes were elevated in large spines suggesting an initial burst of local protein synthesis, and by 2 h only those spines with further enlarged synapses contained polyribosomes. Rapid induction of synaptogenesis was evidenced by an elevation in asymmetric shaft synapses and stubby spines at 5 min and more nonsynaptic filopodia at 30 min. By 2 h, the smallest synaptic spines were markedly reduced in number. This synapse loss was perfectly counterbalanced by enlargement of the remaining excitatory synapses such that the summed synaptic surface area per length of dendritic segment was constant across time and conditions. Remarkably, the inhibitory synapses showed a parallel synaptic plasticity, also demonstrating a decrease in number perfectly counterbalanced by an increase in synaptic surface area. Thus, TBS-LTP triggered spinogenesis followed by loss of small excitatory and inhibitory synapses and a subsequent enlargement of the remaining synapses by 2 h. These data suggest that dendritic segments coordinate structural plasticity across multiple synapses and maintain a homeostatic balance of excitatory and inhibitory inputs through local protein-synthesis and selective capture or redistribution of dendritic resources.
Copyright © 2009 Wiley-Liss, Inc.
Figures



References
-
- Aakalu G, Smith WB, Nguyen N, Jiang C, Schuman EM. Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron. 2001;30:489–502. - PubMed
-
- Abraham WC, Huggett A. Induction and reversal of long-term potentiation by repeated high- frequency stimulation in rat hippocampal slices. Hippocampus. 1997;7:137–145. - PubMed
-
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17:381–386. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

