Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg
- PMID: 20101618
- PMCID: PMC4603937
- DOI: 10.1002/eji.200939782
Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg
Abstract
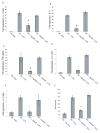
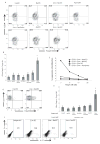
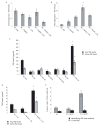
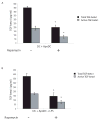
DC apoptosis has been observed in patients with cancer and sepsis, and defects in DC apoptosis have been implicated in the development of autoimmune diseases. However, the mechanisms of how DC apoptosis affects immune responses, are unclear. In this study, we showed that immature viable DC have the ability to uptake apoptotic DC as well as necrotic DC without it being recognized as an inflammatory event by immature viable DC. However, the specific uptake of apoptotic DC converted immature viable DC into tolerogenic DC, which were resistant to LPS-induced maturation. These tolerogenic DC secreted increased levels of TGF-beta1, which induced differentiation of naïve T cells into Foxp3(+) Treg. Furthermore, induction of Treg differentiation only occurred upon uptake of apoptotic DC and not apoptotic splenocytes by viable DC, indicating that it is specifically the uptake of apoptotic DC that gives viable immature DC the potential to induce Foxp3(+) Treg. Taken together, these findings identify uptake of apoptotic DC by viable immature DC as an immunologically tolerogenic event.
Conflict of interest statement
Figures
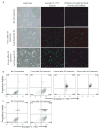
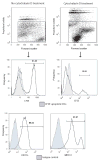
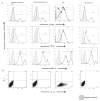
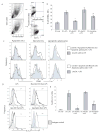
References
-
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. - PubMed
-
- Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

