Pre-existing minority drug-resistant HIV-1 variants, adherence, and risk of antiretroviral treatment failure
- PMID: 20102271
- PMCID: PMC2825289
- DOI: 10.1086/650543
Pre-existing minority drug-resistant HIV-1 variants, adherence, and risk of antiretroviral treatment failure
Abstract
Background: The clinical relevance of detecting minority drug-resistant human immunodeficiency virus type 1 (HIV-1) variants is uncertain.
Methods: To determine the effect of pre-existing minority nonnucleoside reverse-transcriptase inhibitor (NNRTI)-resistant variants on the risk of virologic failure, we reanalyzed a case-cohort substudy of efavirenz recipients in AIDS Clinical Trials Group protocol A5095. Minority K103N or Y181C populations were determined by allele-specific polymerase chain reaction in subjects without NNRTI resistance by population sequencing. Weighted Cox proportional hazards models adjusted for recent treatment adherence estimated the relative risk of virologic failure in the presence of NNRTI-resistant minority variants.
Results: The evaluable case-cohort sample included 195 subjects from the randomly selected subcohort (51 with virologic failure, 144 without virologic failure), plus 127 of the remaining subjects who experienced virologic failure. Presence of minority K103N or Y181C mutations, or both, was detected in 8 (4.4%), 54 (29.5%), and 11 (6%), respectively, of 183 evaluable subjects in the random subcohort. Detection of minority Y181C mutants was associated with an increased risk of virologic failure in the setting of recent treatment adherence (hazard ratio, 3.45 [95% confidence interval, 1.90-6.26]) but not in nonadherent subjects (hazard ratio, 1.39 [95% confidence interval, 0.58-3.29]). Of note, 70% of subjects with minority Y181C variants achieved long-term viral suppression.
Conclusions: In adherent patients, pre-existing minority Y181C mutants more than tripled the risk of virologic failure of first-line efavirenz-based antiretroviral therapy.
Clinical trials registration: NCT00013520.
Figures

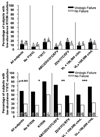

Comment in
-
When do minority drug-resistant HIV-1 variants have a major clinical impact?J Infect Dis. 2010 Mar;201(5):647-9. doi: 10.1086/650545. J Infect Dis. 2010. PMID: 20102273 No abstract available.
References
-
- Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. - PubMed
-
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2008. [January 29, 2008]. Available at http://www.aidsinfo.nih.gov.
-
- Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2008;47:266–285. - PubMed
-
- Kozal MJ, Hullsiek KH, Macarthur RD, et al. The Incidence of HIV drug resistance and its impact on progression of HIV disease among antiretroviral-naive participants started on three different antiretroviral therapy strategies. HIV Clin Trials. 2007;8:357–370. - PubMed
-
- Kuritzkes DR, Lalama CM, Ribaudo HJ, et al. Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis. 2008;197:867–870. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- P30 AI027767/AI/NIAID NIH HHS/United States
- UM1 AI069472/AI/NIAID NIH HHS/United States
- AI069419/AI/NIAID NIH HHS/United States
- AI38858/AI/NIAID NIH HHS/United States
- K24 AI051966/AI/NIAID NIH HHS/United States
- AI069452/AI/NIAID NIH HHS/United States
- AI068636/AI/NIAID NIH HHS/United States
- AI060354/AI/NIAID NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- AI051966/AI/NIAID NIH HHS/United States
- U01 AI069452/AI/NIAID NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- AI06836/AI/NIAID NIH HHS/United States
- AI027767/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- AI069472/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- AI50410/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- RR024996/RR/NCRR NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States

