Myriocin-mediated up-regulation of hepatocyte apoA-I synthesis is associated with ERK inhibition
- PMID: 20102334
- PMCID: PMC2860698
- DOI: 10.1042/CS20090452
Myriocin-mediated up-regulation of hepatocyte apoA-I synthesis is associated with ERK inhibition
Abstract
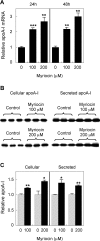
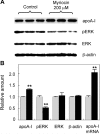
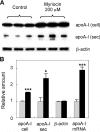
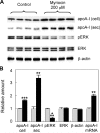
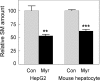
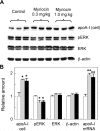
Sphingolipids including sphingomyelin have been implicated as potential atherogenic lipids. Studies in apoE (apolipoprotein E)-null mice have revealed that the serine palmitoyltransferase inhibitor myriocin reduces plasma levels of sphingomyelin, ceramide, sphingosine-1-phosphate and glycosphingolipids and that this is associated with potent inhibition of atherosclerosis. Interestingly, hepatic apoA-I (apolipoprotein A-I) synthesis and plasma HDL (high-density lipoprotein)-cholesterol levels were also increased in apoE-null mice treated with myriocin. Since myriocin is a known inhibitor of ERK (extracellular-signal-related kinase) phosphorylation, we assessed the possibility that myriocin may be acting to increase hepatic apoA-I production via this pathway. To address this, HepG2 cells and primary mouse hepatocytes were treated with 200 muM myriocin for up to 48 h. Myriocin increased apoA-I mRNA and protein levels by approx. 3- and 2-fold respectively. Myriocin also increased apoA-I secretion up to 3.5-fold and decreased ERK phosphorylation by approx. 70%. Similar findings were obtained when primary hepatocytes were isolated from apoE-null mice that were treated with myriocin (intraperitoneal injection at a dose of 0.3 mg/kg body weight). Further experiments revealed that the MEK (mitogen-activated protein kinase/ERK kinase) inhibitor PD98059 potently inhibited ERK phosphorylation, as expected, and increased primary hepatocyte apoA-I production by 3-fold. These results indicate that ERK phosphorylation plays a role in regulating hepatic apoA-I expression and suggest that the anti-atherogenic mechanism of action for myriocin may be linked to this pathway.
Figures


Similar articles
-
Human apolipoprotein A-I induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through ATP-binding cassette transporter A1.Am J Physiol Cell Physiol. 2011 Sep;301(3):C739-48. doi: 10.1152/ajpcell.00055.2011. Epub 2011 Jul 6. Am J Physiol Cell Physiol. 2011. PMID: 21734188
-
Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice.Pharmacol Res. 2008 Jul;58(1):45-51. doi: 10.1016/j.phrs.2008.06.005. Epub 2008 Jun 20. Pharmacol Res. 2008. PMID: 18611440
-
Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration.Biochem Pharmacol. 2007 May 1;73(9):1340-6. doi: 10.1016/j.bcp.2006.12.023. Epub 2006 Dec 27. Biochem Pharmacol. 2007. PMID: 17239824
-
Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice.J Biol Chem. 2005 Mar 18;280(11):10284-9. doi: 10.1074/jbc.M412348200. Epub 2004 Dec 6. J Biol Chem. 2005. PMID: 15590644
-
β3-Adrenergic receptor regulates hepatic apolipoprotein A-I gene expression.J Clin Lipidol. 2017 Sep-Oct;11(5):1168-1176. doi: 10.1016/j.jacl.2017.07.007. Epub 2017 Jul 27. J Clin Lipidol. 2017. PMID: 28802864
Cited by
-
A role for sphingolipids in the pathophysiology of obesity-induced inflammation.Cell Mol Life Sci. 2012 Jul;69(13):2135-46. doi: 10.1007/s00018-012-0917-5. Cell Mol Life Sci. 2012. PMID: 22294100 Free PMC article. Review.
-
Endogenous ceramide contributes to the transcytosis of oxLDL across endothelial cells and promotes its subendothelial retention in vascular wall.Oxid Med Cell Longev. 2014;2014:823071. doi: 10.1155/2014/823071. Epub 2014 Apr 10. Oxid Med Cell Longev. 2014. PMID: 24817993 Free PMC article.
-
ORMDL orosomucoid-like proteins are degraded by free-cholesterol-loading-induced autophagy.Proc Natl Acad Sci U S A. 2015 Mar 24;112(12):3728-33. doi: 10.1073/pnas.1422455112. Epub 2015 Mar 9. Proc Natl Acad Sci U S A. 2015. PMID: 25775599 Free PMC article.
-
Sphingolipids in obesity and related complications.Front Biosci (Landmark Ed). 2017 Jan 1;22(1):96-116. doi: 10.2741/4474. Front Biosci (Landmark Ed). 2017. PMID: 27814604 Free PMC article. Review.
-
Mechanism of the effect of saikosaponin on atherosclerosis in vitro is based on the MAPK signaling pathway.Mol Med Rep. 2017 Dec;16(6):8868-8874. doi: 10.3892/mmr.2017.7691. Epub 2017 Oct 3. Mol Med Rep. 2017. PMID: 28990046 Free PMC article.
References
-
- Ballantyne C. M., Herd J. A., Dunn J. K., Jones P. H., Farmer J. A., Gotto A. M., Jr Effects of lipid lowering therapy on progression of coronary and carotid artery disease. Curr. Opin. Lipidol. 1997;8:354–361. - PubMed
-
- Ballantyne C. M. Rationale for targeting multiple lipid pathways for optimal cardiovascular risk reduction. Am. J. Cardiol. 2005;96:14K–19K. - PubMed
-
- Chatterjee S. Sphingolipids in atherosclerosis and vascular biology. Arterioscler. Thromb. Vasc. Biol. 1998;18:1523–1533. - PubMed
-
- Auge N., Negre-Salvayre A., Salvayre R., Levade T. Sphingomyelin metabolites in vascular cell signaling and atherogenesis. Prog. Lipid. Res. 2000;39:207–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

