p21(Cip1) up-regulated during histone deacetylase inhibitor-induced CD4(+) T-cell anergy selectively associates with mitogen-activated protein kinases
- PMID: 20102411
- PMCID: PMC2842505
- DOI: 10.1111/j.1365-2567.2009.03161.x
p21(Cip1) up-regulated during histone deacetylase inhibitor-induced CD4(+) T-cell anergy selectively associates with mitogen-activated protein kinases
Abstract
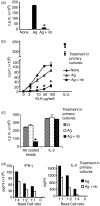
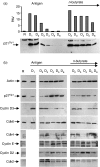
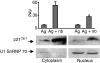
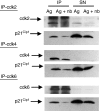
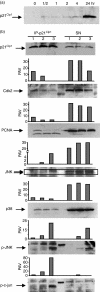
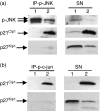
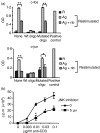
Histone deacetylase inhibitor n-butyrate induced proliferative unresponsiveness in antigen-stimulated murine CD4(+) T cells. T cells anergized by n-butyrate demonstrated reduced interleukin-2 (IL-2) secretion and decreased activating protein 1 (AP-1) activity upon restimulation. Mechanistic studies determined that the cyclin-dependent kinase (cdk) inhibitor p21(Cip1) was up-regulated in the anergic CD4(+) T cells. p21(Cip1) is known to inhibit the cell cycle through its interaction with cdk, proliferating cell nuclear antigen (PCNA) or c-Jun N-terminal kinase (JNK). p21(Cip1) did not preferentially associate with PCNA or cdk in anergic T helper type 1 (Th1) cells. Instead, among the three interaction partners, p21(Cip1) was found to interact with phospho-JNK and phospho-c-jun selectively in the anergic CD4(+) T cells. The activity of c-jun and downstream transcription factor AP-1 were suppressed in the anergic Th1 cells. In contrast, p21(Cip1) and the two phospho-proteins were never detected concurrently in the control CD4(+) T cells. The n-butyrate-induced p21(Cip1)-mediated inhibition of JNK and c-jun represents a novel potential mechanism by which proliferative unresponsiveness was maintained in CD4(+) T cells.
Figures
Similar articles
-
Histone deacetylase inhibitor uses p21(Cip1) to maintain anergy in CD4+ T cells.Int Immunopharmacol. 2009 Oct;9(11):1289-97. doi: 10.1016/j.intimp.2009.07.012. Epub 2009 Aug 5. Int Immunopharmacol. 2009. PMID: 19664724
-
Induction of anergy in Th1 cells associated with increased levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1.J Immunol. 2001 Jan 15;166(2):952-8. doi: 10.4049/jimmunol.166.2.952. J Immunol. 2001. PMID: 11145672
-
The ability of antigen, but not interleukin-2, to promote n-butyrate-induced T helper 1 cell anergy is associated with increased expression and altered association patterns of cyclin-dependent kinase inhibitors.Immunology. 2002 Aug;106(4):486-95. doi: 10.1046/j.1365-2567.2002.01457.x. Immunology. 2002. PMID: 12153511 Free PMC article.
-
In vivo anergized CD4+ T cells have defective expression and function of the activating protein-1 transcription factor.J Immunol. 1998 Dec 1;161(11):5930-6. J Immunol. 1998. PMID: 9834073
-
Histone deacetylase inhibitors: signalling towards p21cip1/waf1.Int J Biochem Cell Biol. 2007;39(7-8):1367-74. doi: 10.1016/j.biocel.2007.03.001. Epub 2007 Mar 7. Int J Biochem Cell Biol. 2007. PMID: 17412634 Review.
Cited by
-
Long circulating lectin conjugated paclitaxel loaded magnetic nanoparticles: a new theranostic avenue for leukemia therapy.PLoS One. 2011;6(11):e26803. doi: 10.1371/journal.pone.0026803. Epub 2011 Nov 16. PLoS One. 2011. PMID: 22110595 Free PMC article.
-
Bone Morphogenetic Proteins Shape Treg Cells.Front Immunol. 2022 Mar 28;13:865546. doi: 10.3389/fimmu.2022.865546. eCollection 2022. Front Immunol. 2022. PMID: 35418975 Free PMC article. Review.
-
Exploring and Verifying the Mechanism and Targets of Shenqi Pill in the Treatment of Nonalcoholic Steatohepatitis via Network Pharmacology and Experiments.J Immunol Res. 2022 Jun 12;2022:6588144. doi: 10.1155/2022/6588144. eCollection 2022. J Immunol Res. 2022. PMID: 35733920 Free PMC article.
-
Procession to pediatric bacteremia and sepsis: covert operations and failures in diplomacy.Pediatrics. 2010 Jul;126(1):137-50. doi: 10.1542/peds.2009-3169. Epub 2010 Jun 21. Pediatrics. 2010. PMID: 20566606 Free PMC article. Review.
-
Suppression of the allogeneic response by the anti-allergy drug N-(3,4-dimethoxycinnamonyl) anthranilic acid results from T-cell cycle arrest.Immunology. 2013 Feb;138(2):157-64. doi: 10.1111/imm.12026. Immunology. 2013. PMID: 23121382 Free PMC article.
References
-
- Gilbert KM, Ernst DN, Hobbs MV, Weigle WO. Effects of tolerance induction on early cell cycle progression by Th1 clones. Cell Immunol. 1992;141:362–72. - PubMed
-
- Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–84. - PubMed
-
- Boussiotis VA, Freeman GJ, Taylor PA, Berezovskaya A, Grass I, Blazar BR, Nadler LM. p27kip1 functions as an anergy factor inhibiting interleukin 2 transcription and clonal expansion of alloreactive human and mouse helper T lymphocytes. Nat Med. 2000;6:290–6. - PubMed
-
- Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–55. - PubMed
-
- Gilbert KM, Weigle WO. Th1 cell anergy and blockade in G1a phase of the cell cycle. J Immunol. 1993;151:1245–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

