Differences in innate immune responses correlate with differences in murine susceptibility to Chlamydia muridarum pulmonary infection
- PMID: 20102413
- PMCID: PMC2842502
- DOI: 10.1111/j.1365-2567.2009.03157.x
Differences in innate immune responses correlate with differences in murine susceptibility to Chlamydia muridarum pulmonary infection
Abstract
We investigated the phenotypic basis for genetically determined differences in susceptibility and resistance to Chlamydia muridarum pulmonary infection using BALB/c and C57BL/6 mice. Following C. muridarum intranasal inoculation, the intensity of infection was very different between BALB/c and C57BL/6 beginning as early as 3 days post-infection. Intrapulmonary cytokine patterns also differed at early time-points (days 2 and 4) between these two strains of mice. The early recruitment of neutrophils to lung tissue was greater in BALB/c than in C57BL/6 mice and correlated with a higher number of inclusion forming units (IFU) of C. muridarum. At day 12 post-infection, BALB/c mice continued to demonstrate a greater burden of infection, significantly higher lung cytokine levels for tumour necrosis factor-alpha and interleukin-17 (IL-17) and a significantly lower level for interferon-gamma than did C57BL/6 mice. In vitro, bone-marrow-derived dendritic cells (BMDCs) from BALB/c mice underwent less functional maturation in response to C. muridarum infection than did BMDCs from C57BL/6 mice. The BMDCs of BALB/c mice expressed lower levels of activation markers (CD80, CD86, CD40 and major histocompatibility complex class II) and secreted less IL-12 and more IL-23 than BMDCs from C57BL/6 mice. Overall, the data demonstrate that the differences exhibited by BALB/c and C57BL/6 mice following C. muridarum pulmonary infection are associated with differences in early innate cytokine and cellular responses that are correlated with late differences in T helper type 17 versus type 1 adaptive immune responses.
Figures
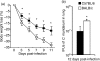
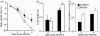




Similar articles
-
Chlamydia muridarum Causes Persistent Subclinical Infection and Elicits Innate and Adaptive Immune Responses in C57BL/6J, BALB/cJ, and J:ARC(S) Mice Following Exposure to Shedding Mice.Comp Med. 2024 Dec 1;74(6):373-391. doi: 10.30802/AALAS-CM-24-057. Comp Med. 2024. PMID: 39853328 Free PMC article.
-
Endogenous IFN-gamma production is induced and required for protective immunity against pulmonary chlamydial infection in neonatal mice.J Immunol. 2008 Mar 15;180(6):4148-55. doi: 10.4049/jimmunol.180.6.4148. J Immunol. 2008. PMID: 18322226
-
The infecting dose of Chlamydia muridarum modulates the innate immune response and ascending infection.Infect Immun. 2004 Nov;72(11):6330-40. doi: 10.1128/IAI.72.11.6330-6340.2004. Infect Immun. 2004. PMID: 15501762 Free PMC article.
-
Role of microRNAs in immune regulation and pathogenesis of Chlamydia trachomatis and Chlamydia muridarum infections: a rapid review.Microbes Infect. 2024 Nov-Dec;26(8):105397. doi: 10.1016/j.micinf.2024.105397. Epub 2024 Jul 17. Microbes Infect. 2024. PMID: 39025257 Review.
-
[The role of cytokines in Chlamydia-induced inflammation].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2025 Jun;41(6):564-570. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2025. PMID: 40525346 Review. Chinese.
Cited by
-
Chlamydia-induced ReA: immune imbalances and persistent pathogens.Nat Rev Rheumatol. 2011 Nov 22;8(1):55-9. doi: 10.1038/nrrheum.2011.173. Nat Rev Rheumatol. 2011. PMID: 22105240
-
Biliatresone induces cholangiopathy in C57BL/6J neonates.Sci Rep. 2023 Jun 29;13(1):10574. doi: 10.1038/s41598-023-37354-z. Sci Rep. 2023. PMID: 37386088 Free PMC article.
-
Different Innate Immune Responses in BALB/c and C57BL/6 Strains following Corneal Transplantation.J Innate Immun. 2021;13(1):49-59. doi: 10.1159/000509716. Epub 2020 Sep 9. J Innate Immun. 2021. PMID: 32906119 Free PMC article.
-
Establishment of head and neck squamous cell carcinoma mouse models for cetuximab resistance and sensitivity.Cancer Drug Resist. 2023 Oct 17;6(4):709-728. doi: 10.20517/cdr.2023.62. eCollection 2023. Cancer Drug Resist. 2023. PMID: 38239393 Free PMC article.
-
Vaccination with Mincle agonist UM-1098 and mycobacterial antigens induces protective Th1 and Th17 responses.NPJ Vaccines. 2024 Jun 6;9(1):100. doi: 10.1038/s41541-024-00897-x. NPJ Vaccines. 2024. PMID: 38844494 Free PMC article.
References
-
- Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia. Intracellular Biology, Pathogenesis, and Immunity. Washington, DC: ASM Press; 1999. pp. 139–69.
-
- Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–61. - PubMed
-
- Zhang D, Yang X, Lu H, Zhong G, Brunham RC. Immunity to Chlamydia trachomatis mouse pneumonitis induced by vaccination with live organisms correlates with early granulocyte-macrophage colony-stimulating factor and interleukin-12 production and with dendritic cell-like maturation. Infect Immun. 1999;67:1606–13. - PMC - PubMed
-
- Eko FO, He Q, Brown T, et al. A novel recombinant multisubunit vaccine against Chlamydia. J Immunol. 2004;173:3375–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

