Interleukin-10 and interferon-gamma modulate surface expression of fractalkine-receptor (CX(3)CR1) via PI3K in monocytes
- PMID: 20102414
- PMCID: PMC2842506
- DOI: 10.1111/j.1365-2567.2009.03181.x
Interleukin-10 and interferon-gamma modulate surface expression of fractalkine-receptor (CX(3)CR1) via PI3K in monocytes
Abstract
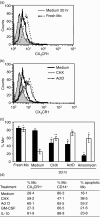
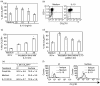
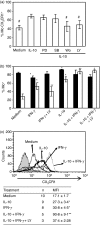
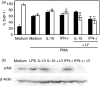
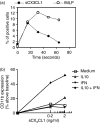
The membrane-anchored form of the chemokine fractalkine (CX(3)CL1) has been identified as a novel adhesion molecule that interacts with its specific receptor (CX(3)CR1) expressed in monocytes, T cells and natural killer cells to induce adhesion. In addition, CX(3)CL1 can be cleaved from the cell membrane to induce chemotaxis of CX(3)CR1-expressing leucocytes. Recently, marked variations in CX(3)CR1 monocyte expression have been observed during several pathological conditions. Regulation of CX(3)CR1 in monocytes during basal or inflammatory/anti-inflammatory conditions is poorly understood. The aim of this study was therefore to examine CX(3)CR1 expression during monocyte maturation and the effect of soluble mediators on this process. We found that basal expression of CX(3)CR1 in fresh monocytes was reduced during culture, and that lipopolysacchairde accelerated this effect. In contrast, interleukin-10 and interferon-gamma treatment abrogated CX(3)CR1 down-modulation, through a phosphatidylinositol 3 kinase-dependent pathway. Most importantly, CX(3)CR1 membrane expression correlated with monocyte CX(3)CL1-dependent function. Taken together, our data demonstrate that CX(3)CR1 expression in monocytes can be modulated, and suggest that alterations in their environment are able to influence CX(3)CL1-dependent functions, such as chemotaxis and adhesion, leading to changes in the kinetics, composition and/or functional status of the leucocyte infiltrate.
Figures
Similar articles
-
The role of CX₃CL1/CX₃CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome.J Hepatol. 2012 Oct;57(4):752-8. doi: 10.1016/j.jhep.2012.05.014. Epub 2012 May 29. J Hepatol. 2012. PMID: 22659346 Free PMC article.
-
CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival.Blood. 2009 Jan 22;113(4):963-72. doi: 10.1182/blood-2008-07-170787. Epub 2008 Oct 29. Blood. 2009. PMID: 18971423
-
High-density lipoproteins suppress chemokines and chemokine receptors in vitro and in vivo.Arterioscler Thromb Vasc Biol. 2010 Sep;30(9):1773-8. doi: 10.1161/ATVBAHA.110.211342. Epub 2010 Aug 11. Arterioscler Thromb Vasc Biol. 2010. PMID: 20702809
-
CX3CL1/fractalkine is a novel regulator of normal and malignant human B cell function.J Leukoc Biol. 2012 Jul;92(1):51-8. doi: 10.1189/jlb.0112035. Epub 2012 Mar 27. J Leukoc Biol. 2012. PMID: 22457367 Review.
-
Selective Dependence of Kidney Dendritic Cells on CX3CR1--Implications for Glomerulonephritis Therapy.Adv Exp Med Biol. 2015;850:55-71. doi: 10.1007/978-3-319-15774-0_5. Adv Exp Med Biol. 2015. PMID: 26324346 Review.
Cited by
-
HIV-1 Tat disrupts CX3CL1-CX3CR1 axis in microglia via the NF-κBYY1 pathway.Curr HIV Res. 2014;12(3):189-200. doi: 10.2174/1570162x12666140526123119. Curr HIV Res. 2014. PMID: 24862326 Free PMC article.
-
Differential expression of the fractalkine chemokine receptor (CX3CR1) in human monocytes during differentiation.Cell Mol Immunol. 2015 Nov;12(6):669-80. doi: 10.1038/cmi.2014.116. Epub 2014 Dec 15. Cell Mol Immunol. 2015. PMID: 25502213 Free PMC article.
-
Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro.Brain Behav Immun. 2013 Aug;32:70-85. doi: 10.1016/j.bbi.2013.02.005. Epub 2013 Feb 27. Brain Behav Immun. 2013. PMID: 23454862 Free PMC article.
-
Polymerization of misfolded Z alpha-1 antitrypsin protein lowers CX3CR1 expression in human PBMCs.Elife. 2021 May 18;10:e64881. doi: 10.7554/eLife.64881. Elife. 2021. PMID: 34002692 Free PMC article.
-
Immunological differences between colorectal cancer and normal mucosa uncover a prognostically relevant immune cell profile.Oncoimmunology. 2018 Nov 5;8(2):e1537693. doi: 10.1080/2162402X.2018.1537693. eCollection 2019. Oncoimmunology. 2018. PMID: 30713795 Free PMC article.
References
-
- Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–4. - PubMed
-
- Umehara H, Tanaka M, Sawaki T, et al. Fractalkine in rheumatoid arthritis and allied conditions. Mod Rheumatol. 2006;16:124–30. - PubMed
-
- Goda S, Imai T, Yoshie O, et al. CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol. 2000;164:4313–20. - PubMed
-
- Hundhausen C, Misztela D, Berkhout TA, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell–cell adhesion. Blood. 2003;102:1186–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

