Evaluation of localized and systemic immune responses in cutaneous leishmaniasis caused by Leishmania tropica: interleukin-8, monocyte chemotactic protein-1 and nitric oxide are major regulatory factors
- PMID: 20102417
- PMCID: PMC2878464
- DOI: 10.1111/j.1365-2567.2009.03223.x
Evaluation of localized and systemic immune responses in cutaneous leishmaniasis caused by Leishmania tropica: interleukin-8, monocyte chemotactic protein-1 and nitric oxide are major regulatory factors
Abstract
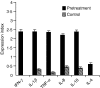
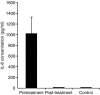
We have established Leishmania tropica as the causative agent of cutaneous leishmaniasis (CL) in the region of India where the disease is endemic. The association between localized and circulating levels of immune-determinants in CL patients was evaluated. Reverse transcription-polymerase chain reaction analysis revealed up-regulation of interferon-gamma (IFN-gamma), interleukin (IL)-1beta, IL-8, tumour necrosis factor-alpha (TNF-alpha), IL-10 and IL-4 in dermal lesions at the pretreatment stage (n = 31) compared with healthy controls (P < 0.001) and a significant down-regulation after treatment (n = 14, P < 0.05). The results indicated that an unfavourable clinical outcome in CL was not related to an inadequate T helper 1 (Th1) cell response, but rather to impairment in multiple immune functions. Comparative assessment of treatment regimes with rifampicin (RFM) or sodium antimony gluconate (SAG) revealed tissue cytokine levels to be significantly reduced after treatment with RFM (P < 0.005), while no significant decrease was evident in the levels of IFN-gamma, TNF-alpha and IL-10 (P > 0.05) as a result of treatment with SAG. Increased transcripts of monocyte chemoattractant protein-1 (MCP-1) (P < 0.001) and inducible nitric oxide synthase (iNOS) (P < 0.05) were evident before treatment in tissue lesions and remained high after treatment. Immunohistochemistry demonstrated strong expression of myeloperoxidase (MPO) and IL-8, and moderate expression of iNOS in dermal lesions. The expression levels of IL-8, MCP-1 and nitric oxide (NO) were high in patient sera before treatment, as determined using cytokine bead array and enzyme-linked immunosorbent assay (ELISA). At the post-treatment stage, the serum IL-8 levels had decreased; however, the levels of MCP-1 and NO remained high. These data suggest that IL-8 is an effector immune-determinant in the progression of CL, whereas NO facilitates the parasite killing by macrophages via MCP-1-mediated stimulation.
Figures



Similar articles
-
An Insight Into Systemic Immune Response in Leishmania donovani Mediated Atypical Cutaneous Leishmaniasis in the New Endemic State of Himachal Pradesh, India.Front Immunol. 2022 Jan 4;12:765684. doi: 10.3389/fimmu.2021.765684. eCollection 2021. Front Immunol. 2022. PMID: 35087516 Free PMC article. Clinical Trial.
-
Correlation of parasitic load with interleukin-4 response in patients with cutaneous leishmaniasis due to Leishmania tropica.FEMS Immunol Med Microbiol. 2009 Dec;57(3):239-46. doi: 10.1111/j.1574-695X.2009.00607.x. Epub 2009 Sep 4. FEMS Immunol Med Microbiol. 2009. PMID: 19799628
-
Analysis of localized immune responses reveals presence of Th17 and Treg cells in cutaneous leishmaniasis due to Leishmania tropica.BMC Immunol. 2013 Nov 22;14:52. doi: 10.1186/1471-2172-14-52. BMC Immunol. 2013. PMID: 24267152 Free PMC article.
-
T(H)1 cells control themselves by producing interleukin-10.Nat Rev Immunol. 2007 Jun;7(6):425-8. doi: 10.1038/nri2097. Nat Rev Immunol. 2007. PMID: 17525751 Review.
-
Host-Directed Therapies for Cutaneous Leishmaniasis.Front Immunol. 2021 Mar 26;12:660183. doi: 10.3389/fimmu.2021.660183. eCollection 2021. Front Immunol. 2021. PMID: 33841444 Free PMC article. Review.
Cited by
-
Caffeic acid combined with autoclaved Leishmania major boosted the protection of infected BALB/c mice by enhancing IgG2 production, IFN-γ/TGF-β and iNO synthase/arginase1 ratios, and the death of infected phagocytes.Inflammopharmacology. 2018 Apr;26(2):621-634. doi: 10.1007/s10787-017-0399-z. Epub 2017 Oct 7. Inflammopharmacology. 2018. PMID: 28988279
-
Hypoxia-Inducible Factor-1 Alpha Stabilization in Human Macrophages during Leishmania major Infection Is Impaired by Parasite Virulence.Korean J Parasitol. 2022 Oct;60(5):317-325. doi: 10.3347/kjp.2022.60.5.317. Epub 2022 Oct 21. Korean J Parasitol. 2022. PMID: 36320108 Free PMC article.
-
Molecular signatures of anthroponotic cutaneous leishmaniasis in the lesions of patients infected with Leishmania tropica.Sci Rep. 2020 Oct 1;10(1):16198. doi: 10.1038/s41598-020-72671-7. Sci Rep. 2020. PMID: 33004861 Free PMC article.
-
An Insight Into Systemic Immune Response in Leishmania donovani Mediated Atypical Cutaneous Leishmaniasis in the New Endemic State of Himachal Pradesh, India.Front Immunol. 2022 Jan 4;12:765684. doi: 10.3389/fimmu.2021.765684. eCollection 2021. Front Immunol. 2022. PMID: 35087516 Free PMC article. Clinical Trial.
-
Clinical and epidemiological characteristics of cutaneous leishmaniasis in Sri Lanka.BMC Infect Dis. 2018 Mar 6;18(1):108. doi: 10.1186/s12879-018-2999-7. BMC Infect Dis. 2018. PMID: 29510669 Free PMC article.
References
-
- Gramiccia M, Gradoni L. The current status of zoonotic leishmaniasis and approaches to disease control. Int J Parasitol. 2005;35:1169–80. - PubMed
-
- Schwenkenbecher JM, Wirth T, Schnur LF, et al. Microsatellite analysis reveals genetic structure of Leishmania tropica. Int J Parasitol. 2006;36:237–46. - PubMed
-
- Kumar R, Bumb RA, Ansari NA, Mehta RD, Salotra P. Cutaneous leishmaniasis caused by Leishmania tropica in Bikaner, India: parasite identification and characterization using molecular and immunological tools. Am J Trop Med Hyg. 2007;76:896–901. - PubMed
-
- Louis J, Himmelrich H, Parra-Lopez C, Tacchini-Cottier F, Launois P. Regulation of protective immunity against Leishmania major in mice. Curr Opin Immunol. 1998;10:459–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

