MicroRNAs: master regulators of ethanol abuse and toxicity?
- PMID: 20102566
- PMCID: PMC2925252
- DOI: 10.1111/j.1530-0277.2009.01126.x
MicroRNAs: master regulators of ethanol abuse and toxicity?
Abstract
Ethanol exerts complex effects on human physiology and health. Ethanol is not only addictive, but it is also a fetal teratogen, an adult neurotoxin, and an etiologic agent in hepatic and cardiovascular disease, inflammation, bone loss, and fracture susceptibility. A large number of genes and signaling mechanisms have been implicated in ethanol's deleterious effects leading to the suggestion that ethanol is a "dirty drug." An important question is, are there cellular "master-switches" that can explain these pleiotropic effects of ethanol? MicroRNAs (miRNAs) have been recently identified as master regulators of the cellular transcriptome and proteome. miRNAs play an increasingly appreciated and crucial role in shaping the differentiation and function of tissues and organs in both health and disease. This critical review discusses new evidence showing that ethanol-sensitive miRNAs are indeed regulatory master-switches. More specifically, miRNAs control the development of tolerance, a crucial component of ethanol addiction. Other drugs of abuse also target some ethanol-sensitive miRNAs suggesting that common biochemical mechanisms underlie addiction. This review also discusses evidence that miRNAs mediate several ethanol pathologies, including disruption of neural stem cell proliferation and differentiation in the exposed fetus, gut leakiness that contributes to endotoxemia and alcoholic liver disease, and possibly also hepatocellular carcinomas and other gastrointestinal cancers. Finally, this review provides a perspective on emerging investigations into potential roles of miRNAs as mediators of ethanol's effects on inflammation and fracture healing, as well as the potential for miRNAs as diagnostic biomarkers and as targets for therapeutic interventions for alcohol-related disorders.
Figures
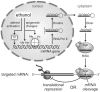

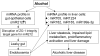
References
-
- Adachi J, Mizoi Y, Fukunaga T, Ogawa Y, Ueno Y, Imamichi H. Degrees of alcohol intoxication in 117 hospitalized cases. J Stud Alcohol. 1991;52(5):448–453. - PubMed
-
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108(1):218–24. - PubMed
-
- Akamatsu W, Okano HJ, Osumi N, Inoue T, Nakamura S, Sakakibara S, Miura M, Matsuo N, Darnell RB, Okano H. Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems. Proc Natl Acad Sci U S A. 1999;96(17):9885–90. - PMC - PubMed
-
- Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113(6):673–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

