KATP channel subunits in rat dorsal root ganglia: alterations by painful axotomy
- PMID: 20102598
- PMCID: PMC2825500
- DOI: 10.1186/1744-8069-6-6
KATP channel subunits in rat dorsal root ganglia: alterations by painful axotomy
Abstract
Background: ATP-sensitive potassium (KATP) channels in neurons mediate neuroprotection, they regulate membrane excitability, and they control neurotransmitter release. Because loss of DRG neuronal KATP currents is involved in the pathophysiology of pain after peripheral nerve injury, we characterized the distribution of the KATP channel subunits in rat DRG, and determined their alterations by painful axotomy using RT-PCR, immunohistochemistry and electron microscopy.
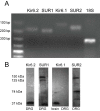
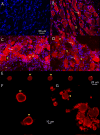
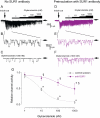
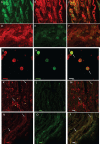
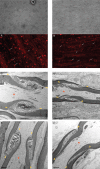
Results: PCR demonstrated Kir6.1, Kir6.2, SUR1 and SUR2 transcripts in control DRG neurons. Protein expression for all but Kir6.1 was confirmed by Western blots and immunohistochemistry. Immunostaining of these subunits was identified by fluorescent and confocal microscopy in plasmalemmal and nuclear membranes, in the cytosol, along the peripheral fibers, and in satellite glial cells. Kir6.2 co-localized with SUR1 subunits. Kir6.2, SUR1, and SUR2 subunits were identified in neuronal subpopulations, categorized by positive or negative NF200 or CGRP staining. KATP current recorded in excised patches was blocked by glybenclamide, but preincubation with antibody against SUR1 abolished this blocking effect of glybenclamide, confirming that the antibody targets the SUR1 protein in the neuronal plasmalemmal membrane. In the myelinated nerve fibers we observed anti-SUR1 immunostaining in regularly spaced funneled-shaped structures. These structures were identified by electron microscopy as Schmidt-Lanterman incisures (SLI) formed by the Schwann cells. Immunostaining against SUR1 and Kir6.2 colocalized with anti-Caspr at paranodal sites.DRG excised from rats made hyperalgesic by spinal nerve ligation exhibited similar staining against Kir6.2, SUR1 or SUR2 as DRG from controls, but showed decreased prevalence of SUR1 immunofluorescent NF200 positive neurons. In DRG and dorsal roots proximal to axotomy SLI were smaller and showed decreased SUR1 immunofluorescence.
Conclusions: We identified Kir6.2/SUR1 and Kir6.2/SUR2 KATP channels in rat DRG neuronal somata, peripheral nerve fibers, and glial satellite and Schwann cells, in both normal state and after painful nerve injury. This is the first report of KATP channels in paranodal sites adjacent to nodes of Ranvier and in the SLI of the Schwann cells. After painful axotomy KATP channels are downregulated in large, myelinated somata and also in SLI, which are also of smaller size compared to controls.Because KATP channels may have diverse functional roles in neurons and glia, further studies are needed to explore the potential of KATP channels as targets of therapies against neuropathic pain and neurodegeneration.
Figures


Similar articles
-
Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: action by direct S-nitrosylation.Mol Pain. 2009 Mar 14;5:12. doi: 10.1186/1744-8069-5-12. Mol Pain. 2009. PMID: 19284878 Free PMC article.
-
ATP-sensitive potassium currents in rat primary afferent neurons: biophysical, pharmacological properties, and alterations by painful nerve injury.Neuroscience. 2009 Aug 18;162(2):431-43. doi: 10.1016/j.neuroscience.2009.04.076. Epub 2009 May 5. Neuroscience. 2009. PMID: 19422886
-
KATP channel subunits are expressed in the epididymal epithelium in several mammalian species.Biol Reprod. 2008 Aug;79(2):253-61. doi: 10.1095/biolreprod.107.064659. Epub 2008 Apr 23. Biol Reprod. 2008. PMID: 18434629
-
Sulfonylurea receptor 1 subunits of ATP-sensitive potassium channels and myocardial ischemia/reperfusion injury.Trends Cardiovasc Med. 2009 Feb;19(2):61-7. doi: 10.1016/j.tcm.2009.04.008. Trends Cardiovasc Med. 2009. PMID: 19577714 Free PMC article. Review.
-
Cardiac sarcolemmal K(ATP) channels: Latest twists in a questing tale!J Mol Cell Cardiol. 2010 Jan;48(1):71-5. doi: 10.1016/j.yjmcc.2009.07.002. Epub 2009 Jul 14. J Mol Cell Cardiol. 2010. PMID: 19607836 Free PMC article. Review.
Cited by
-
SUR1, newly expressed in astrocytes, mediates neuropathic pain in a mouse model of peripheral nerve injury.Mol Pain. 2021 Jan-Dec;17:17448069211006603. doi: 10.1177/17448069211006603. Mol Pain. 2021. PMID: 33788643 Free PMC article.
-
Effects of pinacidil on changes to the microenvironment around the incision site, of a skin/muscle incision and retraction, in a rat model of postoperative pain.Mol Med Rep. 2015 Jul;12(1):829-36. doi: 10.3892/mmr.2015.3465. Epub 2015 Mar 10. Mol Med Rep. 2015. PMID: 25760986 Free PMC article.
-
Functional roles of ATP-sensitive potassium channel as related to anesthesia.J Anesth. 2012 Feb;26(1):152-5. doi: 10.1007/s00540-011-1286-1. Epub 2011 Nov 19. J Anesth. 2012. PMID: 22101773 No abstract available.
-
Preclinical Insights into the Role of Kir4.1 in Chronic Pain and Depression: Mechanisms and Therapeutic Potential.Biomolecules. 2025 Jan 23;15(2):165. doi: 10.3390/biom15020165. Biomolecules. 2025. PMID: 40001468 Free PMC article. Review.
-
Peripheral G protein-coupled inwardly rectifying potassium channels are involved in δ-opioid receptor-mediated anti-hyperalgesia in rat masseter muscle.Eur J Pain. 2014 Jan;18(1):29-38. doi: 10.1002/j.1532-2149.2013.00343.x. Epub 2013 Jun 6. Eur J Pain. 2014. PMID: 23740773 Free PMC article.
References
-
- Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

