Inactivation of respiratory syncytial virus by zinc finger reactive compounds
- PMID: 20102602
- PMCID: PMC2823672
- DOI: 10.1186/1743-422X-7-20
Inactivation of respiratory syncytial virus by zinc finger reactive compounds
Abstract
Background: Infectivity of retroviruses such as HIV-1 and MuLV can be abrogated by compounds targeting zinc finger motif in viral nucleocapsid protein (NC), involved in controlling the processivity of reverse transcription and virus infectivity. Although a member of a different viral family (Pneumoviridae), respiratory syncytial virus (RSV) contains a zinc finger protein M2-1 also involved in control of viral polymerase processivity. Given the functional similarity between the two proteins, it was possible that zinc finger-reactive compounds inactivating retroviruses would have a similar effect against RSV by targeting RSV M2-1 protein. Moreover, inactivation of RSV through modification of an internal protein could yield a safer whole virus vaccine than that produced by RSV inactivation with formalin which modifies surface proteins.
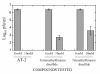
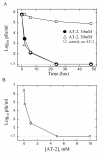
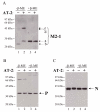
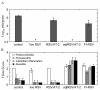
Results: Three compounds were evaluated for their ability to reduce RSV infectivity: 2,2'-dithiodipyridine (AT-2), tetraethylthiuram disulfide and tetramethylthiuram disulfide. All three were capable of inactivating RSV, with AT-2 being the most potent. The mechanism of action of AT-2 was analyzed and it was found that AT-2 treatment indeed results in the modification of RSV M2-1. Altered intramolecular disulfide bond formation in M2-1 protein of AT-2-treated RSV virions might have been responsible for abrogation of RSV infectivity. AT-2-inactivated RSV was found to be moderately immunogenic in the cotton rats S.hispidus and did not cause a vaccine-enhancement seen in animals vaccinated with formalin-inactivated RSV. Increasing immunogenicity of AT-2-inactivated RSV by adjuvant (Ribi), however, led to vaccine-enhanced disease.
Conclusions: This work presents evidence that compounds that inactivate retroviruses by targeting the zinc finger motif in their nucleocapsid proteins are also effective against RSV. AT-2-inactivated RSV vaccine is not strongly immunogenic in the absence of adjuvants. In the adjuvanted form, however, vaccine induces immunopathologic response. The mere preservation of surface antigens of RSV, therefore may not be sufficient to produce a highly-efficacious inactivated virus vaccine that does not lead to an atypical disease.
Figures
References
-
- Clemens R, Safary A, Hepburn A, Roche C, Stanbury WJ, Andre FE. Clinical experience with an inactivated hepatitis A vaccine. J Infect Dis. 1995;171(Suppl 1):S44–9. - PubMed
-
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89(4):422–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

