Growth inhibition of androgen-responsive prostate cancer cells with brefeldin A targeting cell cycle and androgen receptor
- PMID: 20102617
- PMCID: PMC2843609
- DOI: 10.1186/1423-0127-17-5
Growth inhibition of androgen-responsive prostate cancer cells with brefeldin A targeting cell cycle and androgen receptor
Abstract
Background: Androgen ablation is one of the viable therapeutic options for patients with primary hormone (androgen)-dependent prostate cancer. However, an antibiotic brefeldin A (BFA) has been shown to exhibit the growth inhibitory effect on human cancer cells. We thus investigated if BFA might inhibit proliferation of androgen-responsive prostate cancer LNCaP cells and also explored how it would be carried out, focusing on cell cycle and androgen receptor (AR).
Methods: Androgen-mediated cellular events in LNCaP cells were induced using 5alpha-dihydrotestosterone (DHT) as an androgenic mediator. Effects of BFA on non-DHT-stimulated or DHT-stimulated cell growth were assessed. Its growth inhibitory mechanism(s) was further explored; performing cell cycle analysis on a flow cytometer, assessing AR activity by AR binding assay, and analyzing AR protein expression using Western blot analysis.
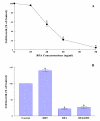
Results: DHT (1 nM) was capable of stimulating LNCaP cell growth by ~40% greater than non-stimulated controls, whereas BFA (30 ng/ml) completely inhibited such DHT-stimulated proliferation. Cell cycle analysis showed that this BFA-induced growth inhibition was associated with a ~75% reduction in the cell number in the S phase and a concomitant increase in the G1 cell number, indicating a G1 cell cycle arrest. This was further confirmed by the modulations of specific cell cycle regulators (CDK2, CDK4, cyclin D1, and p21WAF1), revealed by Western blots. In addition, the growth inhibition induced by BFA was accompanied by a profound (~90%) loss in AR activity, which would be presumably attributed to the significantly reduced cellular AR protein level.
Conclusions: This study demonstrates that BFA has a potent growth inhibitory activity, capable of completely inhibiting DHT (androgen)-stimulated LNCaP proliferation. Such inhibitory action of BFA appears to target cell cycle and AR: BFA led to a G1 cell cycle arrest and the down-regulation of AR activity/expression, possibly accounting for its primary growth inhibitory mechanism. Thus, it is conceivable that BFA may provide a more effective therapeutic modality for patients with hormone-dependent prostate cancer.
Figures



Similar articles
-
Attenuation of androgenic regulation by brefeldin A in androgen-responsive prostate cancer cells.Urol Oncol. 2013 Jan;31(1):104-9. doi: 10.1016/j.urolonc.2010.11.009. Epub 2011 Jul 27. Urol Oncol. 2013. PMID: 21795077
-
Involvement of p27Kip1 in G1 arrest by high dose 5 alpha-dihydrotestosterone in LNCaP human prostate cancer cells.Oncogene. 2000 Feb 3;19(5):670-9. doi: 10.1038/sj.onc.1203369. Oncogene. 2000. PMID: 10698512
-
Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest.Mol Endocrinol. 1998 Jul;12(7):941-53. doi: 10.1210/mend.12.7.0136. Mol Endocrinol. 1998. PMID: 9658399
-
AR, the cell cycle, and prostate cancer.Nucl Recept Signal. 2008 Feb 1;6:e001. doi: 10.1621/nrs.06001. Nucl Recept Signal. 2008. PMID: 18301781 Free PMC article. Review.
-
DNA licensing as a novel androgen receptor mediated therapeutic target for prostate cancer.Endocr Relat Cancer. 2009 Jun;16(2):325-32. doi: 10.1677/ERC-08-0205. Epub 2009 Feb 24. Endocr Relat Cancer. 2009. PMID: 19240183 Free PMC article. Review.
Cited by
-
PSMD4 regulates the malignancy of esophageal cancer cells by suppressing endoplasmic reticulum stress.Kaohsiung J Med Sci. 2019 Oct;35(10):591-597. doi: 10.1002/kjm2.12093. Epub 2019 Jun 4. Kaohsiung J Med Sci. 2019. PMID: 31162820 Free PMC article.
-
Onco-Golgi: Is Fragmentation a Gate to Cancer Progression?Biochem Mol Biol J. 2015;1(1):16. doi: 10.21767/2471-8084.100006. Epub 2015 Nov 7. Biochem Mol Biol J. 2015. PMID: 27064441 Free PMC article.
-
Brefeldin A enhances docetaxel-induced growth inhibition and apoptosis in prostate cancer cells in monolayer and 3D cultures.Bioorg Med Chem Lett. 2017 Jun 1;27(11):2286-2291. doi: 10.1016/j.bmcl.2017.04.047. Epub 2017 Apr 17. Bioorg Med Chem Lett. 2017. PMID: 28462831 Free PMC article.
-
The DNA/RNA helicase DHX9 contributes to the transcriptional program of the androgen receptor in prostate cancer.J Exp Clin Cancer Res. 2022 May 19;41(1):178. doi: 10.1186/s13046-022-02384-4. J Exp Clin Cancer Res. 2022. PMID: 35590370 Free PMC article.
-
Brefeldin a effectively inhibits cancer stem cell-like properties and MMP-9 activity in human colorectal cancer Colo 205 cells.Molecules. 2013 Aug 22;18(9):10242-53. doi: 10.3390/molecules180910242. Molecules. 2013. PMID: 23973996 Free PMC article.
References
-
- Simetal JA, Sar M, Lane MV, French FS, Wilson EM. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–518. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

