The chitobiose transporter, chbC, is required for chitin utilization in Borrelia burgdorferi
- PMID: 20102636
- PMCID: PMC2845121
- DOI: 10.1186/1471-2180-10-21
The chitobiose transporter, chbC, is required for chitin utilization in Borrelia burgdorferi
Abstract
Background: The bacterium Borrelia burgdorferi, the causative agent of Lyme disease, is a limited-genome organism that must obtain many of its biochemical building blocks, including N-acetylglucosamine (GlcNAc), from its tick or vertebrate host. GlcNAc can be imported into the cell as a monomer or dimer (chitobiose), and the annotation for several B. burgdorferi genes suggests that this organism may be able to degrade and utilize chitin, a polymer of GlcNAc. We investigated the ability of B. burgdorferi to utilize chitin in the absence of free GlcNAc, and we attempted to identify genes involved in the process. We also examined the role of RpoS, one of two alternative sigma factors present in B. burgdorferi, in the regulation of chitin utilization.
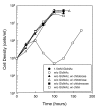
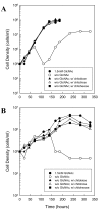
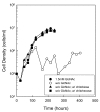
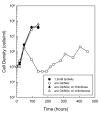
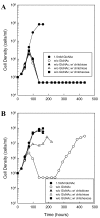
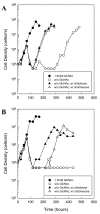
Results: Using fluorescent chitinase substrates, we demonstrated an inherent chitinase activity in rabbit serum, a component of the B. burgdorferi growth medium (BSK-II). After inactivating this activity by boiling, we showed that wild-type cells can utilize chitotriose, chitohexose or coarse chitin flakes in the presence of boiled serum and in the absence of free GlcNAc. Further, we replaced the serum component of BSK-II with a lipid extract and still observed growth on chitin substrates without free GlcNAc. In an attempt to knockout B. burgdorferi chitinase activity, we generated mutations in two genes (bb0002 and bb0620) predicted to encode enzymes that could potentially cleave the beta-(1,4)-glycosidic linkages found in chitin. While these mutations had no effect on the ability to utilize chitin, a mutation in the gene encoding the chitobiose transporter (bbb04, chbC) did block utilization of chitin substrates by B. burgdorferi. Finally, we provide evidence that chitin utilization in an rpoS mutant is delayed compared to wild-type cells, indicating that RpoS may be involved in the regulation of chitin degradation by this organism.
Conclusions: The data collected in this study demonstrate that B. burgdorferi can utilize chitin as a source of GlcNAc in the absence of free GlcNAc, and suggest that chitin is cleaved into dimers before being imported across the cytoplasmic membrane via the chitobiose transporter. In addition, our data suggest that the enzyme(s) involved in chitin degradation are at least partially regulated by the alternative sigma factor RpoS.
Figures
Similar articles
-
Chitobiose utilization in Borrelia burgdorferi is dually regulated by RpoD and RpoS.BMC Microbiol. 2009 May 27;9:108. doi: 10.1186/1471-2180-9-108. BMC Microbiol. 2009. PMID: 19473539 Free PMC article.
-
Study of the response regulator Rrp1 reveals its regulatory role in chitobiose utilization and virulence of Borrelia burgdorferi.Infect Immun. 2013 May;81(5):1775-87. doi: 10.1128/IAI.00050-13. Epub 2013 Mar 11. Infect Immun. 2013. PMID: 23478317 Free PMC article.
-
Genetics and regulation of chitobiose utilization in Borrelia burgdorferi.J Bacteriol. 2001 Oct;183(19):5544-53. doi: 10.1128/JB.183.19.5544-5553.2001. J Bacteriol. 2001. PMID: 11544216 Free PMC article.
-
N-acetylglucosamine: production and applications.Mar Drugs. 2010 Sep 15;8(9):2493-516. doi: 10.3390/md8092493. Mar Drugs. 2010. PMID: 20948902 Free PMC article. Review.
-
N-Acetylglucosamine Sensing and Metabolic Engineering for Attenuating Human and Plant Pathogens.Bioengineering (Basel). 2022 Feb 5;9(2):64. doi: 10.3390/bioengineering9020064. Bioengineering (Basel). 2022. PMID: 35200417 Free PMC article. Review.
Cited by
-
Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle.PLoS Pathog. 2011 Jul;7(7):e1002102. doi: 10.1371/journal.ppat.1002102. Epub 2011 Jul 7. PLoS Pathog. 2011. PMID: 21750672 Free PMC article.
-
Analysis of the BadR regulon in Borrelia burgdorferi.BMC Microbiol. 2025 Feb 26;25(1):94. doi: 10.1186/s12866-025-03797-9. BMC Microbiol. 2025. PMID: 40011802 Free PMC article.
-
MicroRNA and mRNA Transcriptome Profiling in Primary Human Astrocytes Infected with Borrelia burgdorferi.PLoS One. 2017 Jan 30;12(1):e0170961. doi: 10.1371/journal.pone.0170961. eCollection 2017. PLoS One. 2017. PMID: 28135303 Free PMC article.
-
Physiological levels of glucose induce membrane vesicle secretion and affect the lipid and protein composition of Yersinia pestis cell surfaces.Appl Environ Microbiol. 2013 Jul;79(14):4509-14. doi: 10.1128/AEM.00675-13. Epub 2013 May 17. Appl Environ Microbiol. 2013. PMID: 23686263 Free PMC article.
-
Cyclic Di-GMP receptor PlzA controls virulence gene expression through RpoS in Borrelia burgdorferi.Infect Immun. 2014 Jan;82(1):445-52. doi: 10.1128/IAI.01238-13. Epub 2013 Nov 11. Infect Immun. 2014. PMID: 24218478 Free PMC article.
References
-
- Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme Disease - United States, 1992-2006. MMWR Surveill Summ. 2008;57(10):1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

