SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol
- PMID: 20102704
- PMCID: PMC2830376
- DOI: 10.1016/j.bbrc.2010.01.080
SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol
Abstract
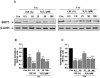
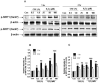
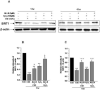
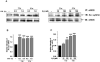
Endothelial nitric oxide synthase (eNOS) plays a crucial role in endothelial cell functions. SIRT1, a NAD(+)-dependent deacetylase, is shown to regulate endothelial function and hence any alteration in endothelial SIRT1 will affect normal vascular physiology. Cigarette smoke (CS)-mediated oxidative stress is implicated in endothelial dysfunction. However, the role of SIRT1 in regulation of eNOS by CS and oxidants are not known. We hypothesized that CS-mediated oxidative stress downregulates SIRT1 leading to acetylation of eNOS which results in reduced nitric oxide (NO)-mediated signaling and endothelial dysfunction. Human umbilical vein endothelial cells (HUVECs) exposed to cigarette smoke extract (CSE) and H(2)O(2) showed decreased SIRT1 levels, activity, but increased phosphorylation concomitant with increased eNOS acetylation. Pre-treatment of endothelial cells with resveratrol significantly attenuated the CSE- and oxidant-mediated SIRT1 levels and eNOS acetylation. These findings suggest that CS- and oxidant-mediated reduction of SIRT1 is associated with acetylation of eNOS which have implications in endothelial dysfunction.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation.J Atheroscler Thromb. 2010 Sep 30;17(9):970-9. doi: 10.5551/jat.4333. Epub 2010 Jul 13. J Atheroscler Thromb. 2010. PMID: 20644332
-
Curcumin Attenuates Hydrogen Peroxide-Induced Premature Senescence via the Activation of SIRT1 in Human Umbilical Vein Endothelial Cells.Biol Pharm Bull. 2015;38(8):1134-41. doi: 10.1248/bpb.b15-00012. Biol Pharm Bull. 2015. PMID: 26235577
-
Polydatin inhibits the oxidative stress-induced proliferation of vascular smooth muscle cells by activating the eNOS/SIRT1 pathway.Int J Mol Med. 2016 Jun;37(6):1652-60. doi: 10.3892/ijmm.2016.2554. Epub 2016 Apr 11. Int J Mol Med. 2016. PMID: 27081912
-
Resveratrol and endothelial nitric oxide.Molecules. 2014 Oct 9;19(10):16102-21. doi: 10.3390/molecules191016102. Molecules. 2014. PMID: 25302702 Free PMC article. Review.
-
Resveratrol as a gene regulator in the vasculature.Curr Pharm Biotechnol. 2014;15(4):401-8. doi: 10.2174/1389201015666140711114450. Curr Pharm Biotechnol. 2014. PMID: 25022271 Review.
Cited by
-
Progress in the Preclinical and Clinical Study of Resveratrol for Vascular Metabolic Disease.Molecules. 2022 Nov 3;27(21):7524. doi: 10.3390/molecules27217524. Molecules. 2022. PMID: 36364370 Free PMC article. Review.
-
Comparative study of nanostructured carriers of calcium phosphate and magnesium phosphate loaded with SRT1720 for the protection of H2O2-induced senescent endothelium.Am J Transl Res. 2018 Jul 15;10(7):2068-2077. eCollection 2018. Am J Transl Res. 2018. PMID: 30093944 Free PMC article.
-
Promotion of nitric oxide production: mechanisms, strategies, and possibilities.Front Physiol. 2025 Jan 23;16:1545044. doi: 10.3389/fphys.2025.1545044. eCollection 2025. Front Physiol. 2025. PMID: 39917079 Free PMC article. Review.
-
Redox regulation of SIRT1 in inflammation and cellular senescence.Free Radic Biol Med. 2013 Aug;61:95-110. doi: 10.1016/j.freeradbiomed.2013.03.015. Epub 2013 Mar 27. Free Radic Biol Med. 2013. PMID: 23542362 Free PMC article. Review.
-
Resveratrol and Cardiovascular Diseases.Nutrients. 2016 May 2;8(5):250. doi: 10.3390/nu8050250. Nutrients. 2016. PMID: 27144581 Free PMC article. Review.
References
-
- Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. - PubMed
-
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–1737. - PubMed
-
- Le Brocq M, Leslie SJ, Milliken P, Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1631–1674. - PubMed
-
- Raij L, DeMaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J Hypertens. 2001;19:891–897. - PubMed
-
- Su Y, Cao W, Han Z, Block ER. Cigarette smoke extract inhibits angiogenesis of pulmonary artery endothelial cells: the role of calpain. Am J Physiol Lung Cell Mol Physiol. 2004;287:L794–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

