Erythropoietin-induced neuroprotection requires cystine glutamate exchanger activity
- PMID: 20102705
- PMCID: PMC2840049
- DOI: 10.1016/j.brainres.2010.01.040
Erythropoietin-induced neuroprotection requires cystine glutamate exchanger activity
Erratum in
- Brain Res. 2010 Nov 29;1362:160
Abstract
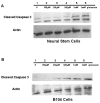
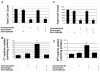
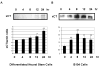
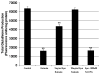
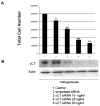
Erythropoietin (Epo) has been used for many years in neonates for the treatment of anemia of prematurity. Epo has also been proposed for treatment of neonatal brain injury, as mounting evidence suggests neuroprotective properties for Epo. However, Epo's neuroprotective mechanism of action is poorly understood. In this study we hypothesized that Epo may confer neuroprotection by enhancing cellular redox defense brought about by cellular glutathione (GSH). This was examined in cultures of differentiated cortical neural stem cells and using the B104 cell line as model systems. Our data shows that Epo causes a time- and dose-dependent increase in expression and activity of system Xc(-), the transporter responsible for uptake of cystine for the production of glutathione. Cystine uptake increases 3-5 fold in differentiated neural stem cells and B104 cells treated with Epo. Exposure of cells to 100 microM kainate suppressed cellular GSH and caused excitotoxicity, but GSH levels and cell viability were completely restored by Epo in the continued presence of kainate. This rescue effect of Epo vanished if system Xc(-) was inhibited pharmacologically using S4-CPG in the presence of Epo leading to marked cell death of B104 cells and cultured mouse cortical neural stem cells. This could also be achieved using xCT siRNA to decrease xCT expression. This data suggests that system Xc(-) activity and protein expression are positively regulated by Epo directly explaining its neuroprotective effect.
2010 Elsevier B.V. All rights reserved.
Figures

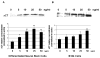
Similar articles
-
Astrocytic cystine/glutamate antiporter is a key regulator of erythropoietin expression in the ischemic retina.FASEB J. 2019 May;33(5):6045-6054. doi: 10.1096/fj.201802144R. Epub 2019 Feb 11. FASEB J. 2019. PMID: 30742774
-
Cystine glutamate exchanger upregulation by retinoic acid induces neuroprotection in neural stem cells.Neuroreport. 2011 Aug 24;22(12):598-602. doi: 10.1097/WNR.0b013e3283494359. Neuroreport. 2011. PMID: 21716153
-
Erythropoietin-induced cytoprotection in intestinal epithelial cells is linked to system Xc<sup/>Exp Cell Res. 2017 Mar 15;352(2):202-206. doi: 10.1016/j.yexcr.2017.02.002. Epub 2017 Feb 6. Exp Cell Res. 2017. PMID: 28167131
-
Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target.CNS Neurol Disord Drug Targets. 2010 Jul;9(3):373-82. doi: 10.2174/187152710791292567. CNS Neurol Disord Drug Targets. 2010. PMID: 20053169 Review.
-
Role of erythropoietin in the brain.Crit Rev Oncol Hematol. 2007 Nov;64(2):159-71. doi: 10.1016/j.critrevonc.2007.03.001. Epub 2007 May 4. Crit Rev Oncol Hematol. 2007. PMID: 17482474 Free PMC article. Review.
Cited by
-
Age-associated expression of erythropoietin and its receptor in rat spiral ganglion neurons and its association with neuronal apoptosis and hearing alterations.Mol Med Rep. 2017 Jan;15(1):228-234. doi: 10.3892/mmr.2016.6010. Epub 2016 Dec 9. Mol Med Rep. 2017. PMID: 27959434 Free PMC article.
-
Regulation of System xc(-) by Pharmacological Manipulation of Cellular Thiols.Oxid Med Cell Longev. 2015;2015:269371. doi: 10.1155/2015/269371. Epub 2015 Apr 9. Oxid Med Cell Longev. 2015. PMID: 25949770 Free PMC article.
-
The transient receptor potential (TRP) channel TRPC3 TRP domain and AMP-activated protein kinase binding site are required for TRPC3 activation by erythropoietin.J Biol Chem. 2011 Sep 2;286(35):30636-30646. doi: 10.1074/jbc.M111.238360. Epub 2011 Jul 14. J Biol Chem. 2011. PMID: 21757714 Free PMC article.
-
Whether Erythropoietin can be a Neuroprotective Agent against Premature Brain Injury: Cellular Mechanisms and Clinical Efficacy.Curr Neuropharmacol. 2022 Mar 4;20(3):611-629. doi: 10.2174/1570159X19666210524154519. Curr Neuropharmacol. 2022. PMID: 34030616 Free PMC article. Review.
-
Trpc2 depletion protects red blood cells from oxidative stress-induced hemolysis.Exp Hematol. 2012 Jan;40(1):71-83. doi: 10.1016/j.exphem.2011.09.006. Epub 2011 Sep 14. Exp Hematol. 2012. PMID: 21924222 Free PMC article.
References
-
- Buemi M, et al. Erythropoietin and the brain: from neurodevelopment to neuroprotection. Clin Sci (Lond) 2002;103:275–82. - PubMed
-
- Calapai G, et al. Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol. 2000;401:349–56. - PubMed
-
- Chong ZZ, et al. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003;71:659–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

