Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells
- PMID: 20102715
- PMCID: PMC6101211
- DOI: 10.1053/j.gastro.2010.01.037
Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells
Abstract
Background & aims: Previous studies of pancreatic acinar cells characterized the effects of Ca(2+)-releasing secretagogues and substances, inducing acute pancreatitis on mitochondrial Ca(2+), transmembrane potential, and NAD(P)H, but dynamic measurements of the crucial intracellular adenosine triphosphate (ATP) levels have not been reported. Here we characterized the effects of these agents on ATP levels in the cytosol and mitochondria.
Methods: ATP levels were monitored using cytosolic- or mitochondrial-targeted luciferases.
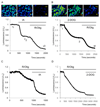
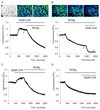
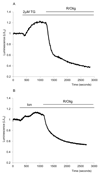
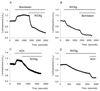
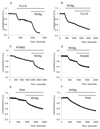
Results: Inhibition of oxidative phosphorylation produced a substantial decrease in cytosolic ATP comparable to that induced by inhibition of glycolysis. Cholecystokinin-8 (CCK) increased cytosolic ATP in spite of accelerating ATP consumption. Acetylcholine, caerulein, and bombesin had similar effect. A bile acid, taurolithocholic acid 3-sulfate (TLC-S); a fatty acid, palmitoleic acid (POA); and palmitoleic acid ethyl ester (POAEE) reduced cytosolic ATP. The ATP decrease in response to these substances was observed in cells with intact or inhibited oxidative phosphorylation. TLC-S, POA, and POAEE reduced mitochondrial ATP, whereas physiological CCK increased mitochondrial ATP. Supramaximal CCK produced a biphasic response composed of a small initial decline followed by a stronger increase.
Conclusions: Both glycolysis and oxidative phosphorylation make substantial contributions to ATP production in acinar cells. Ca(2+)-releasing secretagogues increased ATP level in the cytosol and mitochondria of intact isolated cells. TLC-S, POA, and POAEE reduced cytosolic and mitochondrial ATP. When cells rely on nonoxidative ATP production, secretagogues as well as TLC-S, POA, and POAEE all diminish cytosolic ATP levels.
Copyright 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Barrow SL, Voronina SG, da S X, Chvanov MA, Longbottom RE, Gerasimenko OV, Petersen OH, Rutter GA, Tepikin AV. ATP depletion inhibits Ca2+ release, influx and extrusion in pancreatic acinar cells but not pathological Ca2+ responses induced by bile. Pflugers Arch. 2008;455:1025–1039. - PubMed
-
- Bauduin H, Colin M, Dumont JE. Energy sources for protein synthesis and enzymatic secretion in rat pancreas in vitro. Biochim Biophys Acta. 1969;174:722–733. - PubMed
-
- Bell CJ, Bright NA, Rutter GA, Griffiths EJ. ATP regulation in adult rat cardiomyocytes: time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins. J Biol Chem. 2006;281:28058–28067. - PubMed
-
- Bell CJ, Manfredi G, Griffiths EJ, Rutter GA. Luciferase expression for ATP imaging: application to cardiac myocytes. Methods Cell Biol. 2007;80:341–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

