Effect of adjustable passive constraint on the failing left ventricle: a finite-element model study
- PMID: 20103222
- PMCID: PMC2844501
- DOI: 10.1016/j.athoracsur.2009.08.075
Effect of adjustable passive constraint on the failing left ventricle: a finite-element model study
Abstract
Background: Passive constraint is used to prevent left ventricular dilation and subsequent remodeling. However, there has been concern about the effect of passive constraint on diastolic left ventricular chamber stiffness and pump function. This study determined the relationship between constraint, diastolic wall stress, chamber stiffness, and pump function. We tested the hypothesis that passive constraint at 3 mm Hg reduces wall stress with minimal change in pump function.
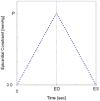
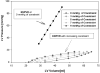
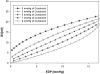
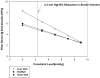
Methods: A three-dimensional finite-element model of the globally dilated left ventricle based on left ventricular dimensions obtained in dogs that had undergone serial intracoronary microsphere injection was created. The model was adjusted to match experimentally observed end-diastolic left ventricular volume and midventricular wall thickness. The experimental results used to create the model were previously reported. A pressure of 3, 5, 7, and 9 mm Hg was applied to the epicardium. Fiber stress, end-diastolic pressure-volume relationship, end-systolic pressure-volume relationship, and the stroke volume-end-diastolic pressure (Starling) relationship were calculated.
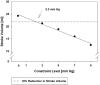
Results: As epicardial constraint pressure increased, fiber stress decreased, the end-diastolic pressure-volume relationship shifted to the left, and the Starling relationship shifted down and to the right. The end-systolic pressure-volume relationship did not change. A constraining pressure of 2.3 mm Hg was associated with a 10% reduction in stroke volume, and mean end-diastolic fiber stress was reduced by 18.3% (inner wall), 15.3% (mid wall), and 14.2% (outer wall).
Conclusions: Both stress and cardiac output decrease in a linear fashion as the amount of passive constraint is increased. If the reduction in cardiac output is to be less than 10%, passive constraint should not exceed 2.3 mm Hg. On the other hand, this amount of constraint may be sufficient to reverse eccentric hypertrophy after myocardial infarction.
2010 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Figures

Comment in
-
Invited commentary.Ann Thorac Surg. 2010 Jan;89(1):137-8. doi: 10.1016/j.athoracsur.2009.09.069. Ann Thorac Surg. 2010. PMID: 20103223 No abstract available.
Similar articles
-
Invited commentary.Ann Thorac Surg. 2010 Jan;89(1):137-8. doi: 10.1016/j.athoracsur.2009.09.069. Ann Thorac Surg. 2010. PMID: 20103223 No abstract available.
-
Residual stress produced by ventricular volume reduction surgery has little effect on ventricular function and mechanics: a finite element model study.J Thorac Cardiovasc Surg. 2001 Sep;122(3):592-9. doi: 10.1067/mtc.2001.114939. J Thorac Cardiovasc Surg. 2001. PMID: 11547315
-
Myosplint decreases wall stress without depressing function in the failing heart: a finite element model study.Ann Thorac Surg. 2003 Oct;76(4):1171-80; discussion 1180. doi: 10.1016/s0003-4975(03)00731-8. Ann Thorac Surg. 2003. PMID: 14530007
-
Reverse remodeling and enhanced inotropic reserve from the cardiac support device in experimental cardiac failure.J Card Fail. 2004 Dec;10(6 Suppl):S215-9. doi: 10.1016/j.cardfail.2004.09.003. J Card Fail. 2004. PMID: 15803553 Review.
-
Assessment of left-ventricular function.Thorac Cardiovasc Surg. 1998 Sep;46 Suppl 2:248-54. doi: 10.1055/s-2007-1013081. Thorac Cardiovasc Surg. 1998. PMID: 9822175 Review.
Cited by
-
Biventricular finite element modeling of the Acorn CorCap Cardiac Support Device on a failing heart.Ann Thorac Surg. 2013 Jun;95(6):2022-7. doi: 10.1016/j.athoracsur.2013.02.032. Epub 2013 May 2. Ann Thorac Surg. 2013. PMID: 23643546 Free PMC article.
-
A coupled biventricular finite element and lumped-parameter circulatory system model of heart failure.Comput Methods Biomech Biomed Engin. 2013;16(8):807-18. doi: 10.1080/10255842.2011.641121. Epub 2012 Jan 16. Comput Methods Biomech Biomed Engin. 2013. PMID: 22248290 Free PMC article.
-
Electromechanical feedback with reduced cellular connectivity alters electrical activity in an infarct injured left ventricle: a finite element model study.Am J Physiol Heart Circ Physiol. 2012 Jan 1;302(1):H206-14. doi: 10.1152/ajpheart.00272.2011. Epub 2011 Nov 4. Am J Physiol Heart Circ Physiol. 2012. PMID: 22058157 Free PMC article.
-
Growth and remodeling of the left ventricle: A case study of myocardial infarction and surgical ventricular restoration.Mech Res Commun. 2012 Jun 1;42:134-141. doi: 10.1016/j.mechrescom.2012.03.005. Epub 2012 Mar 12. Mech Res Commun. 2012. PMID: 22778489 Free PMC article.
-
Optimizing Epicardial Restraint and Reinforcement Following Myocardial Infarction: Moving Towards Localized, Biomimetic, and Multitherapeutic Options.Biomimetics (Basel). 2019 Jan 17;4(1):7. doi: 10.3390/biomimetics4010007. Biomimetics (Basel). 2019. PMID: 31105193 Free PMC article. Review.
References
-
- Anand IS, Liu D, Chugh SS, et al. Isolated myocyte contractile function is normal in postinfarct remodeled rat heart with systolic dysfunction. Circulation. 1997;96(11):3974–84. - PubMed
-
- Hammermeister KE, DeRouen TA, Dodge HT. Variables predictive of survival in patients with coronary disease. Selection by univariate and multivariate analyses from the clinical, electrocardiographic, exercise, arteriographic, and quantitative angiographic evaluations. Circulation. 1979;59(3):421–30. - PubMed
-
- Lee TH, Hamilton MA, Stevenson LW, et al. Impact of left ventricular cavity size on survival in advanced heart failure. Am J Cardiol. 1993;72(9):672–6. - PubMed
-
- Wong M, Johnson G, Shabetai R, et al. Echocardiographic variables as prognostic indicators and therapeutic monitors in chronic congestive heart failure. Veterans Affairs cooperative studies V-HeFT I and II. V-HeFT VA Cooperative Studies Group. Circulation. 1993;87(6 Suppl):VI65–70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

