Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1
- PMID: 20103591
- PMCID: PMC2843194
- DOI: 10.1074/jbc.M109.096842
Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1
Abstract
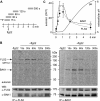
In plants leucine-rich repeat receptor kinases (LRR-RKs) located at the plasma membrane play a pivotal role in the perception of extracellular signals. For two of these LRR-RKs, the brassinosteroid receptor BRI1 and the flagellin receptor FLS2, interaction with the LRR receptor-like kinase BAK1 (BRI1-associated receptor kinase 1) was shown to be required for signal transduction. Here we report that FLS2.BAK1 heteromerization occurs almost instantaneously after perception of the ligand, the flagellin-derived peptide flg22. Flg22 can induce formation of a stable FLS2.BAK1 complex in microsomal membrane preparations in vitro, and the kinase inhibitor K-252a does not prevent complex formation. A kinase dead version of BAK1 associates with FLS2 in a flg22-dependent manner but does not restore responsiveness to flg22 in cells of bak1 plants, demonstrating that kinase activity of BAK1 is essential for FLS2 signaling. Furthermore, using in vivo phospholabeling, we are able to detect de novo phosphorylation of both FLS2 and BAK1 within 15 s of stimulation with flg22. Similarly, brassinolide induces BAK1 phosphorylation within seconds. Other triggers of plant defense, such as bacterial EF-Tu and the endogenous AtPep1 likewise induce rapid formation of heterocomplexes consisting of de novo phosphorylated BAK1 and proteins representing the ligand-specific binding receptors EF-Tu receptor and Pep1 receptor 1, respectively. Thus, we propose that several LRR-RKs form tight complexes with BAK1 almost instantaneously after ligand binding and that the subsequent phosphorylation events are key initial steps in signal transduction.
Figures



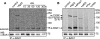
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

