Human mutations that confer paclitaxel resistance
- PMID: 20103599
- PMCID: PMC2820594
- DOI: 10.1158/1535-7163.MCT-09-0674
Human mutations that confer paclitaxel resistance
Abstract
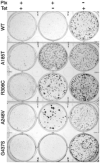
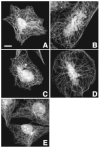
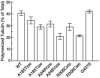
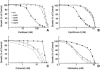
The involvement of tubulin mutations as a cause of clinical drug resistance has been intensely debated in recent years. In the studies described here, we used transfection to test whether beta1-tubulin mutations and polymorphisms found in cancer patients are able to confer resistance to drugs that target microtubules. Three of four mutations (A185T, A248V, R306C, but not G437S) that we tested caused paclitaxel resistance, as indicated by the following observations: (a) essentially 100% of cells selected in paclitaxel contained transfected mutant tubulin; (b) paclitaxel resistance could be turned off using tetracycline to turn off transgene expression; (c) paclitaxel resistance increased as mutant tubulin production increased. All the paclitaxel resistance mutations disrupted microtubule assembly, conferred increased sensitivity to microtubule-disruptive drugs, and produced defects in mitosis. The results are consistent with a mechanism in which tubulin mutations alter microtubule stability in a way that counteracts drug action. These studies show that human tumor cells can acquire spontaneous mutations in beta1-tubulin that cause resistance to paclitaxel, and suggest that patients with some polymorphisms in beta1-tubulin may require higher drug concentrations for effective therapy.
Figures

Similar articles
-
Amino acid substitutions at proline 220 of beta-tubulin confer resistance to paclitaxel and colcemid.Mol Cancer Ther. 2007 Oct;6(10):2798-806. doi: 10.1158/1535-7163.MCT-06-0791. Mol Cancer Ther. 2007. PMID: 17938271
-
Mutations at leucine 215 of beta-tubulin affect paclitaxel sensitivity by two distinct mechanisms.Biochemistry. 2006 Jan 10;45(1):185-94. doi: 10.1021/bi051207d. Biochemistry. 2006. PMID: 16388594
-
Random mutagenesis of β-tubulin defines a set of dispersed mutations that confer paclitaxel resistance.Pharm Res. 2012 Nov;29(11):2994-3006. doi: 10.1007/s11095-012-0794-5. Epub 2012 Jun 6. Pharm Res. 2012. PMID: 22669706
-
Tubulin interacting agents: novel taxanes and epothilones.Curr Oncol Rep. 2003 Mar;5(2):89-98. doi: 10.1007/s11912-003-0095-6. Curr Oncol Rep. 2003. PMID: 12583825 Review.
-
Epothilone B and its analogs - a new family of anticancer agents.Mini Rev Med Chem. 2003 Mar;3(2):149-58. doi: 10.2174/1389557033405269. Mini Rev Med Chem. 2003. PMID: 12570848 Review.
Cited by
-
Enzalutamide therapy for advanced prostate cancer: efficacy, resistance and beyond.Endocr Relat Cancer. 2018 Oct 31;26(1):R31-R52. doi: 10.1530/ERC-18-0289. Endocr Relat Cancer. 2018. PMID: 30382692 Free PMC article.
-
MicroRNAs: key players of taxane resistance and their therapeutic potential in human cancers.J Cell Mol Med. 2013 Oct;17(10):1207-17. doi: 10.1111/jcmm.12131. Epub 2013 Sep 23. J Cell Mol Med. 2013. PMID: 24106980 Free PMC article. Review.
-
Preclinical profile of cabazitaxel.Drug Des Devel Ther. 2014 Oct 13;8:1851-67. doi: 10.2147/DDDT.S64940. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25378905 Free PMC article. Review.
-
Zampanolide, a Microtubule-Stabilizing Agent, Is Active in Resistant Cancer Cells and Inhibits Cell Migration.Int J Mol Sci. 2017 May 3;18(5):971. doi: 10.3390/ijms18050971. Int J Mol Sci. 2017. PMID: 28467385 Free PMC article.
-
Inhibition of cell migration and cell division correlates with distinct effects of microtubule inhibiting drugs.J Biol Chem. 2010 Oct 15;285(42):32242-50. doi: 10.1074/jbc.M110.160820. Epub 2010 Aug 9. J Biol Chem. 2010. PMID: 20696757 Free PMC article.
References
-
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature Reviews. 2004;4:253–65. - PubMed
-
- Cabral F. Factors determining cellular mechanisms of resistance to antimitotic drugs. Drug Resistance Updates. 2001;3:1–6. - PubMed
-
- Berrieman HK, Lind MJ, Cawkwell L. Do beta-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 2004;5:158–64. - PubMed
-
- Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Internatl Rev Cytol. 1998;178:207–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

