Stability of cyclophosphamide in extemporaneous oral suspensions
- PMID: 20103616
- PMCID: PMC2835970
- DOI: 10.1345/aph.1M578
Stability of cyclophosphamide in extemporaneous oral suspensions
Abstract
Background: Cyclophosphamide, an alkylating agent, is widely used for the treatment of many adult and pediatric malignancies. The stability of cyclophosphamide in aqueous- and methylcellulose-based oral suspending vehicles is currently unknown.
Objective: To develop and validate a stability-indicating high-performance liquid chromatography (HPLC) method to measure cyclophosphamide concentrations in simple syrup and Ora-Plus, and assess the 56-day chemical stability and physical appearance of cyclophosphamide in these suspensions at both room temperature (22 degrees C) and 4 degrees C.
Methods: The intravenous formulation of cyclophosphamide was diluted to 20 mg/mL in NaCl 0.9%, compounded 1:1 with either suspending vehicle, and stored in the dark in 3-mL amber polypropylene oral syringes at 4 degrees C and 22 degrees C. Aliquots from each syringe were obtained on days 0, 3, 7, 14, 21, 28, 35, 42, 49, and 56 and assayed using the validated stability-indicating HPLC-UV method. A C18 analytical column was used to separate cyclophosphamide from the internal standard, ifosfamide, with a mobile phase of 21% acetonitrile in 79% sodium phosphate buffer. The suspension was examined for odor change, visually examined under normal fluorescent light for color change, and examined under a light microscope for evidence of microbial growth.
Results: Samples of cyclophosphamide in both simple syrup and Ora-Plus were stable when kept at 4 degrees C for at least 56 days. At room temperature, cyclophosphamide in simple syrup and Ora-Plus had a shelf life of 8 and 3 days, respectively. No changes in color or odor or evidence of microbial growth were observed.
Conclusions: Cyclophosphamide can be extemporaneously prepared in simple syrup or Ora-Plus and stored for at least 2 months under refrigeration without significant degradation.
Figures

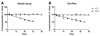
Similar articles
-
Stability of extemporaneously prepared cinacalcet oral suspensions.Am J Health Syst Pharm. 2018 May 1;75(9):e236-e240. doi: 10.2146/ajhp170072. Am J Health Syst Pharm. 2018. PMID: 29691267
-
Stability of dolasetron in two oral liquid vehicles.Am J Health Syst Pharm. 2003 Nov 1;60(21):2242-4. doi: 10.1093/ajhp/60.21.2242. Am J Health Syst Pharm. 2003. PMID: 14619116
-
Stability of extemporaneously prepared glycopyrrolate oral suspensions.Am J Health Syst Pharm. 2011 May 1;68(9):843-5. doi: 10.2146/100247. Am J Health Syst Pharm. 2011. PMID: 21515869
-
Physiochemical and Microbiological Stability of Azathioprine Suspensions in PCCA Base, SuspendIt.Int J Pharm Compd. 2023 Jul-Aug;27(4):330-339. Int J Pharm Compd. 2023. PMID: 37595175 Review.
-
Physicochemical and Microbiological Stability of Compounded Metronidazole Suspensions in PCCA SuspendIt.Int J Pharm Compd. 2021 Mar-Apr;25(2):169-175. Int J Pharm Compd. 2021. PMID: 33798117 Free PMC article. Review.
Cited by
-
Compounded Nonsterile Preparations and FDA-Approved Commercially Available Liquid Products for Children: A North American Update.Pharmaceutics. 2022 May 10;14(5):1032. doi: 10.3390/pharmaceutics14051032. Pharmaceutics. 2022. PMID: 35631618 Free PMC article.
-
Phase I expansion cohort to evaluate the combination of bevacizumab, sorafenib and low-dose cyclophosphamide in children and young adults with refractory or recurrent solid tumours.Eur J Cancer. 2020 Jun;132:35-42. doi: 10.1016/j.ejca.2020.03.010. Epub 2020 Apr 20. Eur J Cancer. 2020. PMID: 32325418 Free PMC article. Clinical Trial.
-
Development of a Stable Lyophilized Cyclophosphamide Monohydrate Formulation Using Non-Aqueous Solvents.AAPS PharmSciTech. 2024 Aug 28;25(7):200. doi: 10.1208/s12249-024-02920-9. AAPS PharmSciTech. 2024. PMID: 39198332
-
Influence of Puncture Devices on the Accuracy of Cyclophosphamide Dosing for Chemotherapy Administration.Pharmaceuticals (Basel). 2025 Jun 12;18(6):879. doi: 10.3390/ph18060879. Pharmaceuticals (Basel). 2025. PMID: 40573273 Free PMC article.
-
Characterization of Pharmaceutical Transformation Products by High-Field Asymmetric Waveform Ion Mobility and Infrared Ion Spectroscopy Coupled to Mass Spectrometry.J Am Soc Mass Spectrom. 2025 Jun 4;36(6):1277-1285. doi: 10.1021/jasms.5c00039. Epub 2025 May 12. J Am Soc Mass Spectrom. 2025. PMID: 40354661 Free PMC article.
References
-
- Ahmed AR, Hombal SM. Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J Am Acad Dermatol. 1984;11:1115–1126. - PubMed
-
- Moore MJ. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 1991;20:194–208. - PubMed
-
- Fleming RA. An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacotherapy. 1997;17:146S–154S. - PubMed
-
- Singh N, Nigam M, Ranjan V, Sharma R, Balapure AK, Rath SK. Caspase mediated enhanced apoptotic action of cyclophosphamide- and resveratrol-treated MCF-7 cells. J Pharmacol Sci. 2009;109:473–485. - PubMed
-
- Sweetenham JW, Carella AM, Taghipour G, et al. High-dose therapy and autologous stem-cell transplantation for adult patients with Hodgkin's disease who do not enter remission after induction chemotherapy: results in 175 patients reported to the European Group for Blood and Marrow Transplantation. Lymphoma Working Party. J Clin Oncol. 1999;17:3101–3109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

