Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenstrom's macroglobulinemia
- PMID: 20103671
- PMCID: PMC2885252
- DOI: 10.1158/1078-0432.CCR-09-1837
Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenstrom's macroglobulinemia
Abstract
Background: Waldenström's macroglobulinemia (WM) is a rare, low-grade lymphoproliferative disorder. Based on preclinical studies, we conducted a phase II clinical trial testing the efficacy and safety of the Akt inhibitor perifosine in patients with relapsed/refractory WM.
Patients and methods: Thirty-seven patients were treated with oral perifosine (150 mg daily) for six cycles. Stable or responding patients were allowed to continue therapy until progression.
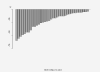
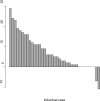
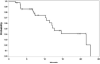
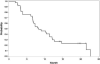
Results: The median age was 65 years (range, 44-82). The median number of prior therapy lines was two (range, one to five). Of the 37 patients, 4 achieved partial response (11%), 9 minimal response (24%), and 20 showed stable disease (54%). The median progression-free survival was 12.6 months. Additionally, beta2 microglobulin of >3.5 mg/dL was associated with poor event-free survival (P = 0.002). Perifosine was generally well tolerated; adverse events related to therapy were cytopenias (grade 3-4, 13%), gastrointestinal symptoms (grade 1-2, 81%), and arthritis flare (all grades, 11%). Translational studies using gene expression profiling and immunohistochemistry showed that perifosine inhibited pGSK activity downstream of Akt, and inhibited nuclear factor kappaB activity.
Conclusion: Perifosine resulted in at least a minimal response in 35% of patients and a median progression-free survival of 12.6 months in patients with relapsed or relapsed/refractory WM, as well as in vivo inhibition of pGSK activity. The results of this study warrant further evaluation of perifosine in combination with rituximab or other active agents in patients with WM.
Figures




Similar articles
-
Waldenström's macroglobulinemia: a clinical perspective in the era of novel therapeutics.Ann Oncol. 2016 Feb;27(2):233-40. doi: 10.1093/annonc/mdv572. Epub 2015 Nov 23. Ann Oncol. 2016. PMID: 26598544 Free PMC article. Review.
-
Combining Ixazomib With Subcutaneous Rituximab and Dexamethasone in Relapsed or Refractory Waldenström's Macroglobulinemia: Final Analysis of the Phase I/II HOVON124/ECWM-R2 Study.J Clin Oncol. 2022 Jan 1;40(1):40-51. doi: 10.1200/JCO.21.00105. Epub 2021 Aug 13. J Clin Oncol. 2022. PMID: 34388022 Free PMC article. Clinical Trial.
-
Two phase 2 trials of the novel Akt inhibitor perifosine in patients with advanced renal cell carcinoma after progression on vascular endothelial growth factor-targeted therapy.Cancer. 2012 Dec 15;118(24):6055-62. doi: 10.1002/cncr.27668. Epub 2012 Jun 6. Cancer. 2012. PMID: 22674198 Free PMC article. Clinical Trial.
-
TAK-228 (formerly MLN0128), an investigational oral dual TORC1/2 inhibitor: A phase I dose escalation study in patients with relapsed or refractory multiple myeloma, non-Hodgkin lymphoma, or Waldenström's macroglobulinemia.Am J Hematol. 2016 Jun;91(4):400-5. doi: 10.1002/ajh.24300. Am J Hematol. 2016. PMID: 26800393 Clinical Trial.
-
Successful treatment of relapsed Waldenström's macroglobulinemia with proteasome inhibitors (bortezomib and subsequently ixazomib) in combination with rituximab and dexamethasone. A case report and review of the of proteasome inhibitors in Waldenström's….Klin Onkol. 2024;37(6):451-462. doi: 10.48095/ccko2024451. Klin Onkol. 2024. PMID: 39772826 Review. English.
Cited by
-
Waldenström Macroglobulinemia: Mechanisms of Disease Progression and Current Therapies.Int J Mol Sci. 2022 Sep 22;23(19):11145. doi: 10.3390/ijms231911145. Int J Mol Sci. 2022. PMID: 36232447 Free PMC article. Review.
-
Novel treatment options for Waldenström macroglobulinemia.Clin Lymphoma Myeloma Leuk. 2013 Sep;13 Suppl 2(0 2):S310-6. doi: 10.1016/j.clml.2013.05.023. Clin Lymphoma Myeloma Leuk. 2013. PMID: 24290218 Free PMC article. Review.
-
Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs.Oncotarget. 2010 Nov;1(7):530-543. doi: 10.18632/oncotarget.188. Oncotarget. 2010. PMID: 21317449 Free PMC article. Review.
-
Impact of informative censoring on the Kaplan-Meier estimate of progression-free survival in phase II clinical trials.J Clin Oncol. 2014 Sep 20;32(27):3068-74. doi: 10.1200/JCO.2014.55.6340. J Clin Oncol. 2014. PMID: 25113767 Free PMC article.
-
Waldenström's macroglobulinemia: a clinical perspective in the era of novel therapeutics.Ann Oncol. 2016 Feb;27(2):233-40. doi: 10.1093/annonc/mdv572. Epub 2015 Nov 23. Ann Oncol. 2016. PMID: 26598544 Free PMC article. Review.
References
-
- Dimopoulos MA, Panayiotidis P, Moulopoulos LA, et al. Waldenstrom's macroglobulinemia: clinical features, complications, and management. J Clin Oncol. 2000;18:214–6. - PubMed
-
- Dimopoulos MA, Kyle RA, Anagnostopoulos A, Treon SP. Diagnosis and management of Waldenstrom's macroglobulinemia. J Clin Oncol. 2005;23:1564–1577. - PubMed
-
- Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, Morra E, Pangalis GA, San Miguel JF, Branagan AR, Dimopoulos MA. Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:110–115. - PubMed
-
- Ghobrial IM, Gertz MA, Fonseca R. Waldenstrom macroglobulinaemia. Lancet Oncol. 2003;4:679–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

